当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiphase Oxidation of Sulfur Dioxide in Aerosol Particles: Implications for Sulfate Formation in Polluted Environments
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.est.0c06496 Tengyu Liu 1, 2 , Arthur W. H. Chan 3 , Jonathan P. D. Abbatt 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-03-24 , DOI: 10.1021/acs.est.0c06496 Tengyu Liu 1, 2 , Arthur W. H. Chan 3 , Jonathan P. D. Abbatt 2
Affiliation
|
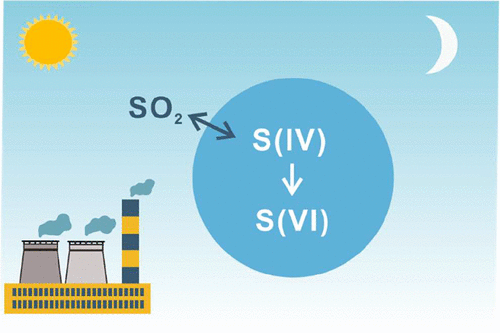
Atmospheric oxidation of sulfur dioxide (SO2) forms sulfate-containing aerosol particles that impact air quality, climate, and human and ecosystem health. It is well-known that in-cloud oxidation of SO2 frequently dominates over gas-phase oxidation on regional and global scales. Multiphase oxidation involving aerosol particles, fog, and cloud droplets has been generally thought to scale with liquid water content (LWC) so multiphase oxidation would be negligible for aerosol particles due to their low aerosol LWC. However, recent field evidence, particularly from East Asia, shows that fast sulfate formation prevails in cloud-free environments that are characterized by high aerosol loadings. By assuming that the kinetics of cloud water chemistry prevails for aerosol particles, most atmospheric models do not capture this phenomenon. Therefore, the field of aerosol SO2 multiphase chemistry has blossomed in the past decade, with many oxidation processes proposed to bridge the difference between modeled and observed sulfate mass loadings. This review summarizes recent advances in the fundamental understanding of the aerosol multiphase oxidation of SO2, with a focus on environmental conditions that affect the oxidation rate, experimental challenges, mechanisms and kinetics results for individual reaction pathways, and future research directions. Compared to dilute cloud water conditions, this paper highlights the differences that arise at the molecular level with the extremely high solute strengths present in aerosol particles.
中文翻译:

气溶胶颗粒中二氧化硫的多相氧化:污染环境中硫酸盐形成的含义。
二氧化硫(SO 2)在大气中的氧化会形成含硫酸盐的气溶胶颗粒,从而影响空气质量,气候以及人类和生态系统的健康。众所周知,SO 2的云内氧化在区域和全球范围内,气相氧化通常占主导地位。通常认为,涉及气溶胶颗粒,雾和云滴的多相氧化会随液态水含量(LWC)缩放,因此,由于其低气溶胶LWC,多相氧化对于气溶胶颗粒而言可以忽略不计。但是,最近的实地证据,尤其是来自东亚的实地证据表明,在以高气溶胶含量为特征的无云环境中,硫酸盐快速形成是普遍的。通过假设气溶胶颗粒占主导地位的是云水化学动力学,大多数大气模型都无法捕捉到这种现象。因此,SO 2气雾剂领域在过去的十年中,多相化学蓬勃发展,提出了许多氧化方法来弥合模拟和观察到的硫酸盐质量负载之间的差异。这篇综述总结了对SO 2气溶胶多相氧化的基本理解的最新进展,重点是影响氧化速率的环境条件,实验挑战,单个反应途径的机理和动力学结果以及未来的研究方向。与稀云水条件相比,本文重点介绍了在气溶胶颗粒中存在的具有极高溶质强度的分子水平上的差异。
更新日期:2021-04-20
中文翻译:

气溶胶颗粒中二氧化硫的多相氧化:污染环境中硫酸盐形成的含义。
二氧化硫(SO 2)在大气中的氧化会形成含硫酸盐的气溶胶颗粒,从而影响空气质量,气候以及人类和生态系统的健康。众所周知,SO 2的云内氧化在区域和全球范围内,气相氧化通常占主导地位。通常认为,涉及气溶胶颗粒,雾和云滴的多相氧化会随液态水含量(LWC)缩放,因此,由于其低气溶胶LWC,多相氧化对于气溶胶颗粒而言可以忽略不计。但是,最近的实地证据,尤其是来自东亚的实地证据表明,在以高气溶胶含量为特征的无云环境中,硫酸盐快速形成是普遍的。通过假设气溶胶颗粒占主导地位的是云水化学动力学,大多数大气模型都无法捕捉到这种现象。因此,SO 2气雾剂领域在过去的十年中,多相化学蓬勃发展,提出了许多氧化方法来弥合模拟和观察到的硫酸盐质量负载之间的差异。这篇综述总结了对SO 2气溶胶多相氧化的基本理解的最新进展,重点是影响氧化速率的环境条件,实验挑战,单个反应途径的机理和动力学结果以及未来的研究方向。与稀云水条件相比,本文重点介绍了在气溶胶颗粒中存在的具有极高溶质强度的分子水平上的差异。






























 京公网安备 11010802027423号
京公网安备 11010802027423号