当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio‐ and Enantioselective Copper‐Catalyzed 1,4‐Conjugate Addition of Trimethylaluminium to Linear α,β,γ,δ‐Unsaturated Alkyl Ketones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-08-01 , DOI: 10.1002/adsc.201600375 Xiaoting Wu 1 , Fang Xie 1 , Zheng Ling 1 , Liang Tang 1 , Wanbin Zhang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2016-08-01 , DOI: 10.1002/adsc.201600375 Xiaoting Wu 1 , Fang Xie 1 , Zheng Ling 1 , Liang Tang 1 , Wanbin Zhang 1
Affiliation
|
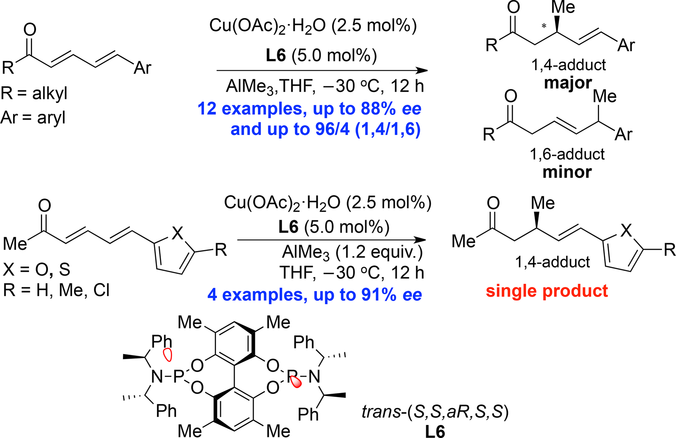
A regio‐ and enantioselective copper‐catalyzed 1,4‐conjugate addition of trimethylaluminium to linear δ‐aryl‐substituted α,β,γ,δ‐unsaturated alkyl ketones was developed. A series of γ,δ‐unsaturated alkyl ketones were obtained in good yields with high regio‐ and enantioselectivity (up to 88% ee and 96:4 dr). Expansion of the reaction scope to substrates containing aromatic heterocycles also afforded good yields and enantioselectivities (up to 91% ee) with very high regioselectivities, exclusively providing the single 1,4‐products.
中文翻译:

区域和对映体选择性铜催化的1,4-共轭三甲基铝与线性α,β,γ,δ-不饱和烷基酮
开发了区域和对映体选择性铜催化的三甲基铝向线性δ-芳基取代的α,β,γ,δ-不饱和烷基酮的1,4-共轭加成反应。以高收率和高区域选择性和对映选择性(高达ee达88%和dr:96:4 )获得了一系列γ,δ-不饱和烷基酮。将反应范围扩大到含有芳族杂环的底物,还具有良好的收率和对映选择性(最高91%ee),具有很高的区域选择性,仅提供单一的1,4-产物。
更新日期:2016-08-01
中文翻译:

区域和对映体选择性铜催化的1,4-共轭三甲基铝与线性α,β,γ,δ-不饱和烷基酮
开发了区域和对映体选择性铜催化的三甲基铝向线性δ-芳基取代的α,β,γ,δ-不饱和烷基酮的1,4-共轭加成反应。以高收率和高区域选择性和对映选择性(高达ee达88%和dr:96:4 )获得了一系列γ,δ-不饱和烷基酮。将反应范围扩大到含有芳族杂环的底物,还具有良好的收率和对映选择性(最高91%ee),具有很高的区域选择性,仅提供单一的1,4-产物。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号