当前位置:
X-MOL 学术
›
Appl. Organomet. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of (4R)-2-(3-hydroxyphenyl)thiazolidine-4-carboxylic acid substituted phthalocyanines: Anticancer activity on different cancer cell lines and molecular docking studies
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-03-26 , DOI: 10.1002/aoc.6242 Ahmet T. Bilgiçli 1 , Hayriye Genc Bilgicli 1 , Ceylan Hepokur 2 , Burak Tüzün 3 , Armağan Günsel 1 , Mustafa Zengin 1 , M. Nilüfer Yarasir 1
Applied Organometallic Chemistry ( IF 3.7 ) Pub Date : 2021-03-26 , DOI: 10.1002/aoc.6242 Ahmet T. Bilgiçli 1 , Hayriye Genc Bilgicli 1 , Ceylan Hepokur 2 , Burak Tüzün 3 , Armağan Günsel 1 , Mustafa Zengin 1 , M. Nilüfer Yarasir 1
Affiliation
|
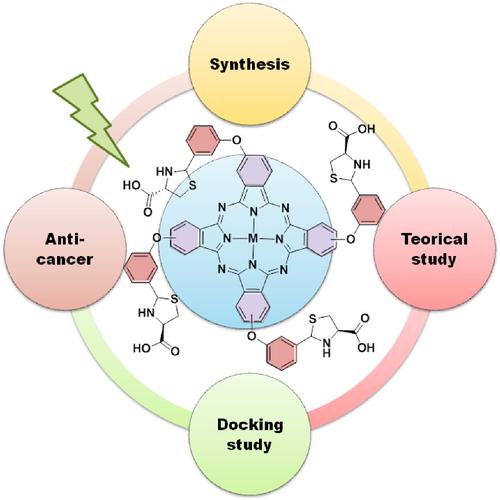
In this study, firstly, (4R)-2-(3-hydroxyphenyl)thiazolidine-4-carboxylic acid (1) and (4R)-2-(3-(3,4-dicyanophenoxy)phenyl)thiazolidine-4-carboxylic acid (2) were prepared. Then, the novel type metallophthalocyanines (ZnPc (3), CuPc (4), and CoPc (5)) bearing thiazolidine groups in peripheral positions were synthesized using by compound (2). The synthesized new compounds (1–5) were characterized by the combination of standard spectroscopic methods such as FT-IR, 1H NMR, 13C NMR, UV–Vis spectral data, and MALDI-TOF. Aggregation behaviors of peripheral tetra-substituted metallophthalocyanines were investigated in dimethyl sulfoxide (DMSO) media. Fluorescence properties and fluorescence quantum yield of the new type zinc phthalocyanine (3) were performed in DMSO at room temperature. The anticancer activity of novel type metallophthalocyanines bearing thiazolidine groups in peripheral positions were investigated on rat glioma cancer (C6), human prostate carcinoma (DU-145), and normal human lung fibroblast (WI-38) cell lines. Finally, the biological and chemical activities of (4R)-2-(3-(3,4-dicyanophenoxy)phenyl)thiazolidine-4-carboxylic acid (2) and its novel type metallophthalocyanines (ZnPc (3), CuPc (4), and CoPc (5)) have been compared with many parameters obtained using theoretical methods that are the Gaussian software and molecular docking.
中文翻译:

(4R)-2-(3-羟基苯基)噻唑烷-4-羧酸取代酞菁的合成:对不同癌细胞系的抗癌活性和分子对接研究
在本研究中,首先,(4R)-2-(3-羟基苯基)噻唑烷-4-羧酸( 1 )和(4R)-2-(3-(3,4-二氰基苯氧基)苯基)噻唑烷-4-羧酸制备酸( 2 )。然后,利用化合物( 2 )合成了在外围位置带有噻唑烷基团的新型金属酞菁(ZnPc( 3 )、CuPc( 4 )和CoPc( 5 ) )。合成的新化合物(1-5)通过标准光谱方法如 FT-IR、1 H NMR、13C NMR、UV-Vis 光谱数据和 MALDI-TOF。在二甲基亚砜 (DMSO) 介质中研究了外围四取代金属酞菁的聚集行为。新型酞菁锌( 3 )的荧光性质和荧光量子产率在DMSO中室温下进行。在大鼠神经胶质瘤 (C6)、人前列腺癌 (DU-145) 和正常人肺成纤维细胞 (WI-38) 细胞系上研究了在外周位置带有噻唑烷基团的新型金属酞菁的抗癌活性。最后,(4R)-2-(3-(3,4-二氰基苯氧基)苯基)噻唑烷-4-羧酸( 2 )及其新型金属酞菁(ZnPc( 3 )、CuPc( 4 )的生物和化学活性)) 和 CoPc ( 5 )) 与使用高斯软件和分子对接等理论方法获得的许多参数进行了比较。
更新日期:2021-03-26
中文翻译:

(4R)-2-(3-羟基苯基)噻唑烷-4-羧酸取代酞菁的合成:对不同癌细胞系的抗癌活性和分子对接研究
在本研究中,首先,(4R)-2-(3-羟基苯基)噻唑烷-4-羧酸( 1 )和(4R)-2-(3-(3,4-二氰基苯氧基)苯基)噻唑烷-4-羧酸制备酸( 2 )。然后,利用化合物( 2 )合成了在外围位置带有噻唑烷基团的新型金属酞菁(ZnPc( 3 )、CuPc( 4 )和CoPc( 5 ) )。合成的新化合物(1-5)通过标准光谱方法如 FT-IR、1 H NMR、13C NMR、UV-Vis 光谱数据和 MALDI-TOF。在二甲基亚砜 (DMSO) 介质中研究了外围四取代金属酞菁的聚集行为。新型酞菁锌( 3 )的荧光性质和荧光量子产率在DMSO中室温下进行。在大鼠神经胶质瘤 (C6)、人前列腺癌 (DU-145) 和正常人肺成纤维细胞 (WI-38) 细胞系上研究了在外周位置带有噻唑烷基团的新型金属酞菁的抗癌活性。最后,(4R)-2-(3-(3,4-二氰基苯氧基)苯基)噻唑烷-4-羧酸( 2 )及其新型金属酞菁(ZnPc( 3 )、CuPc( 4 )的生物和化学活性)) 和 CoPc ( 5 )) 与使用高斯软件和分子对接等理论方法获得的许多参数进行了比较。






























 京公网安备 11010802027423号
京公网安备 11010802027423号