The Journal of Chemical Thermodynamics ( IF 2.2 ) Pub Date : 2021-03-27 , DOI: 10.1016/j.jct.2021.106472
Lan Yang , Xiuxiu Yang , Dan Li , Liwei Zhuang , Lingzong Meng , Tianlong Deng , Yafei Guo
|
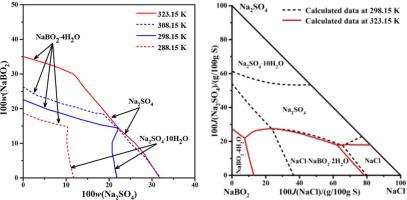
The solubilities and refractive indices in the systems Na2SO4–NaBO2–H2O and NaCl–Na2SO4–NaBO2–H2O at 323.15 K were studied by the isothermal dissolution method. The phase diagram of the ternary system is composed of two single-salt crystallizations for Na2SO4 and NaBO2·4H2O. A comparison of the diagrams of system Na2SO4–NaBO2–H2O from 288.15 K to 323.15 K shows that the crystallization region of NaBO2·4H2O changes greatly as the temperature increasing, and the solid phase of mirabilite at 288.15 K and 298.15 K transforms into the nardite at 308.15 K and 323.15 K. The dry-salt diagram of the quaternary system consists of four salt crystallization fields for NaCl, NaBO2·4H2O, NaCl·NaBO2·2H2O and Na2SO4, five univariant solubility curves and two invariant points. On the basis of the Pitzer model, the lacking Pitzer parameters and dissolution equilibrium constants of the solid phases in this quaternary system were fitted with the solubilities and other thermodynamic properties in this study and literature. The calculated solubilities in the two systems are in good agreement with the experimental data.
中文翻译:

四元体系NaCl–Na 2 SO 4 –NaBO 2 –H 2 O在323.15 K时固液平衡的溶解度测量和热力学模型
用等温溶解法研究了Na 2 SO 4 -NaBO 2 -H 2 O和NaCl-Na 2 SO 4 -NaBO 2 -H 2 O在323.15 K时的溶解度和折射率。三元体系的相图由Na 2 SO 4和NaBO 2 ·4H 2 O的两个单盐结晶组成。系统Na 2 SO 4 –NaBO 2 –H 2 O从288.15 K到323.15 K表明NaBO 2的结晶区域·4H 2 O随着温度的升高而发生很大变化,在288.15 K和298.15 K处的芒硝固相转变为308.15 K和323.15 K的钠钙石。四元体系的干盐图由四个盐结晶场组成。 NaCl,NaBO 2 ·4H 2 O,NaCl·NaBO 2 ·2H 2 O和Na 2 SO 4,五个单变量溶解度曲线和两个不变点。在Pitzer模型的基础上,该四元体系中缺乏Pitzer参数和固相的溶解平衡常数与本研究和文献中的溶解度和其他热力学性质相吻合。在两个系统中计算出的溶解度与实验数据非常吻合。

































 京公网安备 11010802027423号
京公网安备 11010802027423号