当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study of Oxygen-Vacancy Distribution in In2O3
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-03-25 , DOI: 10.1021/acs.jpcc.1c01462
Lu Liu 1 , Chang-Chun He 1 , Jiarui Zeng 1 , Yin-Hui Peng 1 , Wan-Yu Chen 1 , Yu-Jun Zhao 1 , Xiao-Bao Yang 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2021-03-25 , DOI: 10.1021/acs.jpcc.1c01462
Lu Liu 1 , Chang-Chun He 1 , Jiarui Zeng 1 , Yin-Hui Peng 1 , Wan-Yu Chen 1 , Yu-Jun Zhao 1 , Xiao-Bao Yang 1
Affiliation
|
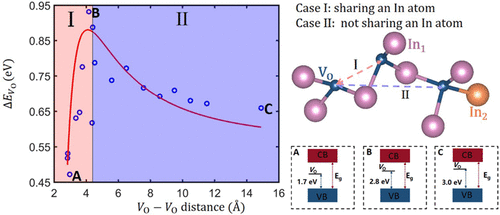
In2O3 is of particular interest as a wide-gap transparent semiconductor oxide, in which the shallow donor defect of the oxygen vacancy plays an important role in electronic properties. Herein, we focus on the oxygen vacancy with various concentrations in In2O3, where the distribution is found to be crucial to the structural stabilities. For a specific supercell, the formation energies of oxygen-vacancy pairs remarkably depend on the distance between the two vacancies, which can be used to determine the oxygen-vacancy distribution in the nonstoichiometric In2O3 structure. Interestingly, when two oxygen vacancies share a same In atom, the structures are approximately stabilized with the decreasing of distance. However, when two oxygen vacancies are not attached to the same In atom, the structures become more stable with the increasing of distance between vacancies. In addition, the gap states induced by oxygen vacancies move toward the valence band maximum (VBM) when the nearest distance between the two vacancies decreases, which will have a great effect on the conductivity.
中文翻译:

In 2 O 3中氧空位分布的理论研究
In 2 O 3作为宽间隙透明半导体氧化物特别令人关注,其中氧空位的浅施主缺陷在电子性质中起重要作用。在这里,我们集中于In 2 O 3中各种浓度的氧空位,发现其分布对结构稳定性至关重要。对于特定的超级电池,氧空位对的形成能显着取决于两个空位之间的距离,这可以用来确定非化学计量的In 2 O 3中的氧空位分布结构体。有趣的是,当两个氧空位共享相同的In原子时,随着距离的减小,结构近似稳定。但是,当两个氧空位未连接到同一In原子上时,随着空位之间距离的增加,结构变得更稳定。另外,当两个空位之间的最近距离减小时,由氧空位引起的间隙状态朝着价带最大值(VBM)移动,这将对电导率产生很大的影响。
更新日期:2021-04-08
中文翻译:

In 2 O 3中氧空位分布的理论研究
In 2 O 3作为宽间隙透明半导体氧化物特别令人关注,其中氧空位的浅施主缺陷在电子性质中起重要作用。在这里,我们集中于In 2 O 3中各种浓度的氧空位,发现其分布对结构稳定性至关重要。对于特定的超级电池,氧空位对的形成能显着取决于两个空位之间的距离,这可以用来确定非化学计量的In 2 O 3中的氧空位分布结构体。有趣的是,当两个氧空位共享相同的In原子时,随着距离的减小,结构近似稳定。但是,当两个氧空位未连接到同一In原子上时,随着空位之间距离的增加,结构变得更稳定。另外,当两个空位之间的最近距离减小时,由氧空位引起的间隙状态朝着价带最大值(VBM)移动,这将对电导率产生很大的影响。































 京公网安备 11010802027423号
京公网安备 11010802027423号