Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced electrocatalytic oxygen reduction reaction for Fe–N4–C by the incorporation of Co nanoparticles
Nanoscale ( IF 5.8 ) Pub Date : 2021-3-13 , DOI: 10.1039/d1nr00727k Tao Jiang 1, 2, 3, 4 , Weiling Luan 1, 2, 3, 4 , Lyudmila Turyanska 5, 6, 7 , Qi Feng 4, 8, 9, 10
Nanoscale ( IF 5.8 ) Pub Date : 2021-3-13 , DOI: 10.1039/d1nr00727k Tao Jiang 1, 2, 3, 4 , Weiling Luan 1, 2, 3, 4 , Lyudmila Turyanska 5, 6, 7 , Qi Feng 4, 8, 9, 10
Affiliation
|
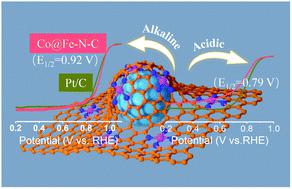
Oxygen reduction reaction (ORR) catalytic activity can be improved by means of enhancing the synergy between transition metals. In this work, a novel porous Fe–N4–C nanostructure containing uniformly dispersed Co nanoparticles (CoNPs) is prepared by an assisted thermal loading method. The as-prepared Co@Fe–N–C catalyst shows enhanced ORR activity with a half-wave potential (E1/2) of 0.92 V vs. RHE, which is much higher than those of the direct pyrolysis CoNP-free sample Fe–N–C (E1/2 = 0.85 V) and Pt/C (E1/2 = 0.90 V) in alkaline media. It exhibits remarkable stability with only a 10 mV decrease in E1/2 after 10 000 cycles and an outstanding long-term durability with 85% current remaining after 60 000 s. In acidic media, this catalyst exhibits catalytic activity with an E1/2 of 0.79 V, comparable to Pt/C (E1/2 = 0.82 V). X-ray absorption fine spectroscopy analysis revealed the presence of active centres of Fe–N4. Density functional theory calculations confirmed the strong synergy between CoNPs and Fe–N4 sites, providing a lower overpotential and beneficial electronic structure and a local coordination environment for the ORR. The incorporation of CoNPs on the surface of Fe–N4–C nanomaterials plays a key role in enhancing the ORR catalytic activity and stability, providing a new route to prepare efficient Pt-free ORR catalysts.
中文翻译:

通过掺入Co纳米颗粒增强Fe–N4–C的电催化氧还原反应
氧还原反应(ORR)的催化活性可以通过增强过渡金属之间的协同作用来提高。在这项工作中,通过辅助热加载方法制备了包含均匀分散的Co纳米颗粒(CoNPs)的新型多孔Fe–N 4 –C纳米结构。所制备的Co @ Fe–N–C催化剂具有增强的ORR活性,相对于RHE的半波电势(E 1/2)为0.92 V ,远高于直接热解的无CoNP样品Fe在碱性介质中为–N–C(E 1/2 = 0.85 V)和Pt / C(E 1/2 = 0.90 V)。它表现出卓越的稳定性,E 1/2仅降低10 mV经过10 000次循环和出色的长期耐用性,在60 000 s后仍保留85%的电流。在酸性介质中,该催化剂的催化活性E 1/2为0.79 V,与Pt / C相当(E 1/2 = 0.82 V)。X射线吸收光谱分析表明,存在Fe–N 4活性中心。密度泛函理论计算证实了CoNP和Fe–N 4位点之间的强协同作用,从而为ORR提供了较低的过电势和有益的电子结构以及局部配位环境。CoNP在Fe–N 4表面的结合-C纳米材料在增强ORR催化活性和稳定性方面起着关键作用,为制备高效的无Pt ORR催化剂提供了新途径。
更新日期:2021-03-25
中文翻译:

通过掺入Co纳米颗粒增强Fe–N4–C的电催化氧还原反应
氧还原反应(ORR)的催化活性可以通过增强过渡金属之间的协同作用来提高。在这项工作中,通过辅助热加载方法制备了包含均匀分散的Co纳米颗粒(CoNPs)的新型多孔Fe–N 4 –C纳米结构。所制备的Co @ Fe–N–C催化剂具有增强的ORR活性,相对于RHE的半波电势(E 1/2)为0.92 V ,远高于直接热解的无CoNP样品Fe在碱性介质中为–N–C(E 1/2 = 0.85 V)和Pt / C(E 1/2 = 0.90 V)。它表现出卓越的稳定性,E 1/2仅降低10 mV经过10 000次循环和出色的长期耐用性,在60 000 s后仍保留85%的电流。在酸性介质中,该催化剂的催化活性E 1/2为0.79 V,与Pt / C相当(E 1/2 = 0.82 V)。X射线吸收光谱分析表明,存在Fe–N 4活性中心。密度泛函理论计算证实了CoNP和Fe–N 4位点之间的强协同作用,从而为ORR提供了较低的过电势和有益的电子结构以及局部配位环境。CoNP在Fe–N 4表面的结合-C纳米材料在增强ORR催化活性和稳定性方面起着关键作用,为制备高效的无Pt ORR催化剂提供了新途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号