当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Click Nucleophilic Conjugate Additions to Activated Alkynes: Exploring Thiol-yne, Amino-yne, and Hydroxyl-yne Reactions from (Bio)Organic to Polymer Chemistry
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-03-25 , DOI: 10.1021/acs.chemrev.0c01076 Joshua C Worch 1 , Connor J Stubbs 1 , Matthew J Price 1 , Andrew P Dove 1
Chemical Reviews ( IF 51.4 ) Pub Date : 2021-03-25 , DOI: 10.1021/acs.chemrev.0c01076 Joshua C Worch 1 , Connor J Stubbs 1 , Matthew J Price 1 , Andrew P Dove 1
Affiliation
|
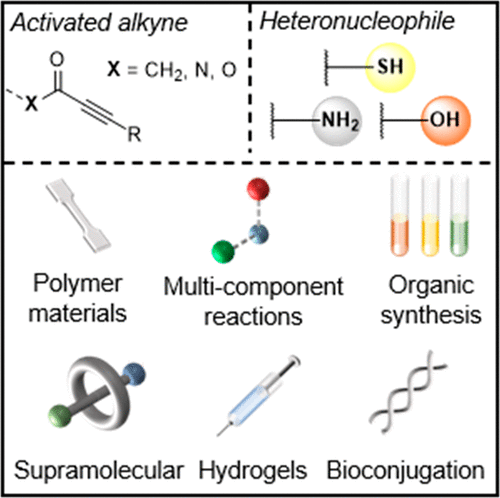
The 1,4-conjugate addition reaction between activated alkynes or acetylenic Michael acceptors and nucleophiles (i.e., the nucleophilic Michael reaction) is a historically useful organic transformation. Despite its general utility, the efficiency and outcomes can vary widely and are often closely dependent upon specific reaction conditions. Nevertheless, with improvements in reaction design, including catalyst development and an expansion of the substrate scope to feature more electrophilic alkynes, many examples now present with features that are congruent with Click chemistry. Although several nucleophilic species can participate in these conjugate additions, ubiquitous nucleophiles such as thiols, amines, and alcohols are commonly employed and, consequently, among the most well developed. For many years, these conjugate additions were largely relegated to organic chemistry, but in the last few decades their use has expanded into other spheres such as bioorganic chemistry and polymer chemistry. Within these fields, they have been particularly useful for bioconjugation reactions and step-growth polymerizations, respectively, due to their excellent efficiency, orthogonality, and ambient reactivity. The reaction is expected to feature in increasingly divergent application settings as it continues to emerge as a Click reaction.
中文翻译:

活化炔烃的点击亲核共轭加成:探索从(生物)有机到聚合物化学的硫醇-炔、氨基-炔和羟基-炔反应
活化的炔烃或炔属迈克尔受体与亲核试剂之间的1,4-共轭加成反应(即亲核迈克尔反应)是历史上有用的有机转化。尽管其具有普遍用途,但效率和结果差异很大,并且通常密切依赖于特定的反应条件。然而,随着反应设计的改进,包括催化剂的开发和底物范围的扩展以具有更多亲电炔烃,现在许多例子都具有与点击化学一致的特征。尽管几种亲核物质可以参与这些共轭加成,但普遍存在的亲核试剂(例如硫醇、胺和醇)是常用的,因此也是最发达的。多年来,这些共轭添加物很大程度上被归入有机化学领域,但在过去的几十年里,它们的用途已扩展到其他领域,例如生物有机化学和高分子化学。在这些领域中,由于其优异的效率、正交性和环境反应性,它们分别特别适用于生物共轭反应和逐步增长聚合。该反应预计将在日益多样化的应用程序设置中出现,因为它继续以点击反应的形式出现。
更新日期:2021-03-25
中文翻译:

活化炔烃的点击亲核共轭加成:探索从(生物)有机到聚合物化学的硫醇-炔、氨基-炔和羟基-炔反应
活化的炔烃或炔属迈克尔受体与亲核试剂之间的1,4-共轭加成反应(即亲核迈克尔反应)是历史上有用的有机转化。尽管其具有普遍用途,但效率和结果差异很大,并且通常密切依赖于特定的反应条件。然而,随着反应设计的改进,包括催化剂的开发和底物范围的扩展以具有更多亲电炔烃,现在许多例子都具有与点击化学一致的特征。尽管几种亲核物质可以参与这些共轭加成,但普遍存在的亲核试剂(例如硫醇、胺和醇)是常用的,因此也是最发达的。多年来,这些共轭添加物很大程度上被归入有机化学领域,但在过去的几十年里,它们的用途已扩展到其他领域,例如生物有机化学和高分子化学。在这些领域中,由于其优异的效率、正交性和环境反应性,它们分别特别适用于生物共轭反应和逐步增长聚合。该反应预计将在日益多样化的应用程序设置中出现,因为它继续以点击反应的形式出现。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号