当前位置:
X-MOL 学术
›
Eur. J. Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrosonium and Nitronium Salts of New Mixed‐Cation Hexafluoridoantimonates(V)
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2021-03-22 , DOI: 10.1002/ejic.202100139
Zoran Mazej 1 , Evgeny Goreshnik 2
European Journal of Inorganic Chemistry ( IF 2.2 ) Pub Date : 2021-03-22 , DOI: 10.1002/ejic.202100139
Zoran Mazej 1 , Evgeny Goreshnik 2
Affiliation
|
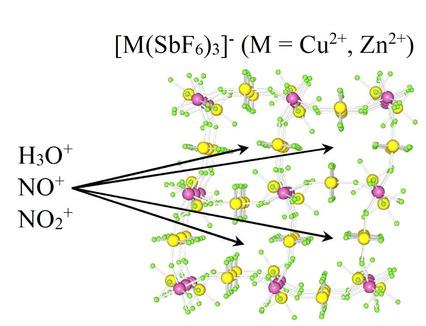
Reaction between NOSbF6 and Cu(SbF6)2 in aHF yielded NOCu(SbF6)3. In the presence of H3OSbF6, single crystals of (H3O)0.2(NO)0.8Cu(SbF6)3 were grown from a saturated aHF solution. Both salts are isotypic and crystallize in the orthorhombic space group Pnma (No. 62) at 200 K. The main feature of their crystal structures are rings of CuF6 octahedra sharing apexes with SbF6 octahedra connected into 3‐D network. Single charged cations are located inside the cavities. Reaction between 0.25NOSbF6, 0.75NO2SbF6 and Cu(SbF6)2 in aHF yielded (NO)0.25(NO2)0.75Cu(SbF6)3 upon the crystallization. It crystallizes in the cubic space group Im (No. 204) at 200 K. Subsequent experiments with M(SbF6)2 (M=Cu, Zn) showed that, when the NO+ cations are partially or completely replaced by H3O+, the grown crystals of (H3O)0.125(NO)0.125(NO2)0.75Zn(SbF6)3 and (H3O)0.25(NO2)0.75M(SbF6)3 (M=Cu, Zn) are isotypic to (NO)0.25(NO2)0.75Cu(SbF6)3. Crystal structure of the latter demonstrates similar 3‐D structural motif as observed for the NOCu(SbF6)3 salt. The crystal structures of (H3O)0.64(NO2)0.36CuF(SbF6)2 and [Cu(H2O)6](SbF6)2 were also determined. Crystallizations of NOSbF6/O2SbF6 were carried out in aHF in different molar ratios. The crystal structure determinations of grown single crystals showed that they have the composition (NO)x(O2)1‐xSbF6 (x=0.17, 0.55 0.75) indicating that most likely all phases with x=0–1 exist. They are isotypic to NOSbF6.
(No. 204) at 200 K. Subsequent experiments with M(SbF6)2 (M=Cu, Zn) showed that, when the NO+ cations are partially or completely replaced by H3O+, the grown crystals of (H3O)0.125(NO)0.125(NO2)0.75Zn(SbF6)3 and (H3O)0.25(NO2)0.75M(SbF6)3 (M=Cu, Zn) are isotypic to (NO)0.25(NO2)0.75Cu(SbF6)3. Crystal structure of the latter demonstrates similar 3‐D structural motif as observed for the NOCu(SbF6)3 salt. The crystal structures of (H3O)0.64(NO2)0.36CuF(SbF6)2 and [Cu(H2O)6](SbF6)2 were also determined. Crystallizations of NOSbF6/O2SbF6 were carried out in aHF in different molar ratios. The crystal structure determinations of grown single crystals showed that they have the composition (NO)x(O2)1‐xSbF6 (x=0.17, 0.55 0.75) indicating that most likely all phases with x=0–1 exist. They are isotypic to NOSbF6.
中文翻译:

新型混合阳离子六氟锑酸盐的氮盐和氮盐(V)
NOSbF 6与Cu(SbF 6)2在aHF中反应生成NOCu(SbF 6)3。在H 3 OSbF 6的存在下,从饱和aHF溶液中生长出(H 3 O)0.2(NO)0.8 Cu(SbF 6)3的单晶。两种盐都是同型的,并且在正交晶空间群Pnma(No. 62)中在200 K时结晶。它们的晶体结构的主要特征是CuF 6八面体的环与SbF 6共享顶点八面体连接到3D网络。单电荷阳离子位于腔体内。0.25NOSbF之间反应6,0.75NO 2的SbF 6和Cu(SBF 6)2中,产生AHF(NO)0.25(NO 2)0.75铜(SBF 6)3时结晶。它 在200 K的立方空间群Im (No. 204)中结晶。随后用M(SbF 6)2(M = Cu,Zn)进行的实验表明,当NO +阳离子被H 3 O部分或完全替代时,+,(H 3 O)0.125(NO)0.125(NO 2)0.75 Zn(SbF 6)3和(H 3 O)0.25(NO 2)0.75 M(SbF 6)3(M = Cu,Zn )与(NO)0.25(NO 2)0.75 Cu(SbF 6)3同型。后者的晶体结构表现出与NOCu(SbF 6)3盐类似的3-D结构基序。(H 3 O)的晶体结构还测定了0.64(NO 2)0.36 CuF(SbF 6)2和[Cu(H 2 O)6 ](SbF 6)2。在aHF中以不同的摩尔比进行NOSbF 6 / O 2 SbF 6的结晶。生长的单晶的晶体结构测定表明,它们的组成为(NO)x(O 2)1-x SbF 6(x = 0.17,0.55 0.75),表明最可能存在x = 0–1的所有相。它们与NOSbF 6是同型的。
(No. 204)中结晶。随后用M(SbF 6)2(M = Cu,Zn)进行的实验表明,当NO +阳离子被H 3 O部分或完全替代时,+,(H 3 O)0.125(NO)0.125(NO 2)0.75 Zn(SbF 6)3和(H 3 O)0.25(NO 2)0.75 M(SbF 6)3(M = Cu,Zn )与(NO)0.25(NO 2)0.75 Cu(SbF 6)3同型。后者的晶体结构表现出与NOCu(SbF 6)3盐类似的3-D结构基序。(H 3 O)的晶体结构还测定了0.64(NO 2)0.36 CuF(SbF 6)2和[Cu(H 2 O)6 ](SbF 6)2。在aHF中以不同的摩尔比进行NOSbF 6 / O 2 SbF 6的结晶。生长的单晶的晶体结构测定表明,它们的组成为(NO)x(O 2)1-x SbF 6(x = 0.17,0.55 0.75),表明最可能存在x = 0–1的所有相。它们与NOSbF 6是同型的。
更新日期:2021-05-10
 (No. 204) at 200 K. Subsequent experiments with M(SbF6)2 (M=Cu, Zn) showed that, when the NO+ cations are partially or completely replaced by H3O+, the grown crystals of (H3O)0.125(NO)0.125(NO2)0.75Zn(SbF6)3 and (H3O)0.25(NO2)0.75M(SbF6)3 (M=Cu, Zn) are isotypic to (NO)0.25(NO2)0.75Cu(SbF6)3. Crystal structure of the latter demonstrates similar 3‐D structural motif as observed for the NOCu(SbF6)3 salt. The crystal structures of (H3O)0.64(NO2)0.36CuF(SbF6)2 and [Cu(H2O)6](SbF6)2 were also determined. Crystallizations of NOSbF6/O2SbF6 were carried out in aHF in different molar ratios. The crystal structure determinations of grown single crystals showed that they have the composition (NO)x(O2)1‐xSbF6 (x=0.17, 0.55 0.75) indicating that most likely all phases with x=0–1 exist. They are isotypic to NOSbF6.
(No. 204) at 200 K. Subsequent experiments with M(SbF6)2 (M=Cu, Zn) showed that, when the NO+ cations are partially or completely replaced by H3O+, the grown crystals of (H3O)0.125(NO)0.125(NO2)0.75Zn(SbF6)3 and (H3O)0.25(NO2)0.75M(SbF6)3 (M=Cu, Zn) are isotypic to (NO)0.25(NO2)0.75Cu(SbF6)3. Crystal structure of the latter demonstrates similar 3‐D structural motif as observed for the NOCu(SbF6)3 salt. The crystal structures of (H3O)0.64(NO2)0.36CuF(SbF6)2 and [Cu(H2O)6](SbF6)2 were also determined. Crystallizations of NOSbF6/O2SbF6 were carried out in aHF in different molar ratios. The crystal structure determinations of grown single crystals showed that they have the composition (NO)x(O2)1‐xSbF6 (x=0.17, 0.55 0.75) indicating that most likely all phases with x=0–1 exist. They are isotypic to NOSbF6.
中文翻译:

新型混合阳离子六氟锑酸盐的氮盐和氮盐(V)
NOSbF 6与Cu(SbF 6)2在aHF中反应生成NOCu(SbF 6)3。在H 3 OSbF 6的存在下,从饱和aHF溶液中生长出(H 3 O)0.2(NO)0.8 Cu(SbF 6)3的单晶。两种盐都是同型的,并且在正交晶空间群Pnma(No. 62)中在200 K时结晶。它们的晶体结构的主要特征是CuF 6八面体的环与SbF 6共享顶点八面体连接到3D网络。单电荷阳离子位于腔体内。0.25NOSbF之间反应6,0.75NO 2的SbF 6和Cu(SBF 6)2中,产生AHF(NO)0.25(NO 2)0.75铜(SBF 6)3时结晶。它 在200 K的立方空间群Im
 (No. 204)中结晶。随后用M(SbF 6)2(M = Cu,Zn)进行的实验表明,当NO +阳离子被H 3 O部分或完全替代时,+,(H 3 O)0.125(NO)0.125(NO 2)0.75 Zn(SbF 6)3和(H 3 O)0.25(NO 2)0.75 M(SbF 6)3(M = Cu,Zn )与(NO)0.25(NO 2)0.75 Cu(SbF 6)3同型。后者的晶体结构表现出与NOCu(SbF 6)3盐类似的3-D结构基序。(H 3 O)的晶体结构还测定了0.64(NO 2)0.36 CuF(SbF 6)2和[Cu(H 2 O)6 ](SbF 6)2。在aHF中以不同的摩尔比进行NOSbF 6 / O 2 SbF 6的结晶。生长的单晶的晶体结构测定表明,它们的组成为(NO)x(O 2)1-x SbF 6(x = 0.17,0.55 0.75),表明最可能存在x = 0–1的所有相。它们与NOSbF 6是同型的。
(No. 204)中结晶。随后用M(SbF 6)2(M = Cu,Zn)进行的实验表明,当NO +阳离子被H 3 O部分或完全替代时,+,(H 3 O)0.125(NO)0.125(NO 2)0.75 Zn(SbF 6)3和(H 3 O)0.25(NO 2)0.75 M(SbF 6)3(M = Cu,Zn )与(NO)0.25(NO 2)0.75 Cu(SbF 6)3同型。后者的晶体结构表现出与NOCu(SbF 6)3盐类似的3-D结构基序。(H 3 O)的晶体结构还测定了0.64(NO 2)0.36 CuF(SbF 6)2和[Cu(H 2 O)6 ](SbF 6)2。在aHF中以不同的摩尔比进行NOSbF 6 / O 2 SbF 6的结晶。生长的单晶的晶体结构测定表明,它们的组成为(NO)x(O 2)1-x SbF 6(x = 0.17,0.55 0.75),表明最可能存在x = 0–1的所有相。它们与NOSbF 6是同型的。































 京公网安备 11010802027423号
京公网安备 11010802027423号