当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Barrierless HONO and HOS(O)2-NO2 Formation via NH3-Promoted Oxidation of SO2 by NO2
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-03-23 , DOI: 10.1021/acs.jpca.1c00539 Guoying Wang 1 , Shangrong Ma 1 , Xiuli Niu 2 , Xuefu Chen 1 , Fengshuo Liu 1 , Xin Li 1 , Lan Li 1 , Gaofeng Shi 1 , Zhijun Wu 3
The Journal of Physical Chemistry A ( IF 2.7 ) Pub Date : 2021-03-23 , DOI: 10.1021/acs.jpca.1c00539 Guoying Wang 1 , Shangrong Ma 1 , Xiuli Niu 2 , Xuefu Chen 1 , Fengshuo Liu 1 , Xin Li 1 , Lan Li 1 , Gaofeng Shi 1 , Zhijun Wu 3
Affiliation
|
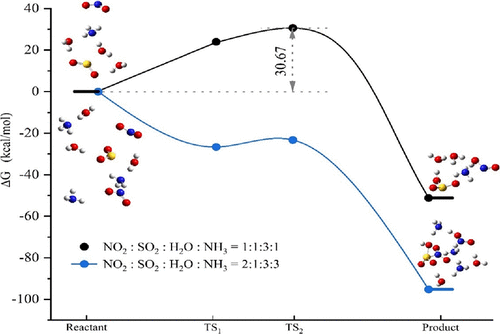
In the troposphere, the knowledge about nitrous acid (HONO) sources is incomplete. The missing source of sulfate and fine particles cannot be explained during haze events. Air quality models cannot predict high levels of secondary fine-particle pollution. Despite extensive studies, one challenging issue in atmospheric chemistry is identifying the source of HONO. Here, we present direct ab initio molecular dynamics simulation evidence and typical air pollution events of the formation of gaseous HONO, nitrogen dioxide/hydrogen sulfite (HOS(O)2-NO2 or NO2-HSO3) from nitrogen dioxide (NO2), sulfur dioxide (SO2), water (H2O), and ammonia (NH3) molecules in a proportion of 2:1:3:3. The reactions show a new mechanism for the formation of HONO and NO2-HSO3 in the troposphere, especially when the concentration of NO2, SO2, H2O, and NH3 is high (e.g., 2:1:3:3 or higher) in the air. Contrary to the proportion NO2, SO2, H2O, and NH3 equaling to 1:1:3:1 and 1:1:3:2, the proportion (2:1:3:3) enables barrierless reactions and weak interactions between molecules via the formation of HONO, NO2-HSO3, and NH3/H2O. In addition, field observations are carried out, and the measured data are summarized. Correlation analysis supported the conversion of NO2 to HONO during observational studies. The weak interactions promote proton transfer, resulting in the generation of HONO, NO2-HSO3, and NH3/H2O pairs.
中文翻译:

Barrierless HONO和HOS(O)2-NO 2经由形成NH 3 SO的促进的氧化2由NO 2
在对流层中,关于亚硝酸(HONO)来源的知识尚不完善。雾霾事件无法解释硫酸盐和细颗粒的缺失来源。空气质量模型无法预测高水平的二次细颗粒物污染。尽管进行了广泛的研究,但大气化学中的一个具有挑战性的问题是确定HONO的来源。在这里,我们提供了从头开始的分子动力学模拟证据,以及由二氧化氮(NO 2)形成气态HONO,二氧化氮/亚硫酸氢盐(HOS(O)2-NO 2或NO 2 -HSO 3)的典型空气污染事件。),二氧化硫(SO 2),水(H 2 O)和氨水(NH 3)分子的比例为2:1:3:3。反应显示出在对流层中形成HONO和NO 2 -HSO 3的新机理,特别是当NO 2,SO 2,H 2 O和NH 3的浓度较高时(例如2:1:3: 3或更高)。与等于1:1:3:1和1:1:3:2的NO 2,SO 2,H 2 O和NH 3的比例相反,比例(2:1:3:3)可以实现无障碍反应,并且分子之间通过HONO,NO 2 -HSO 3和NH 3 / H 2形成的弱相互作用O.另外,进行现场观察,并汇总测量数据。相关性分析支持了观察研究期间NO 2向HONO的转化。弱相互作用促进质子转移,导致生成HONO,NO 2 -HSO 3和NH 3 / H 2 O对。
更新日期:2021-04-01
中文翻译:

Barrierless HONO和HOS(O)2-NO 2经由形成NH 3 SO的促进的氧化2由NO 2
在对流层中,关于亚硝酸(HONO)来源的知识尚不完善。雾霾事件无法解释硫酸盐和细颗粒的缺失来源。空气质量模型无法预测高水平的二次细颗粒物污染。尽管进行了广泛的研究,但大气化学中的一个具有挑战性的问题是确定HONO的来源。在这里,我们提供了从头开始的分子动力学模拟证据,以及由二氧化氮(NO 2)形成气态HONO,二氧化氮/亚硫酸氢盐(HOS(O)2-NO 2或NO 2 -HSO 3)的典型空气污染事件。),二氧化硫(SO 2),水(H 2 O)和氨水(NH 3)分子的比例为2:1:3:3。反应显示出在对流层中形成HONO和NO 2 -HSO 3的新机理,特别是当NO 2,SO 2,H 2 O和NH 3的浓度较高时(例如2:1:3: 3或更高)。与等于1:1:3:1和1:1:3:2的NO 2,SO 2,H 2 O和NH 3的比例相反,比例(2:1:3:3)可以实现无障碍反应,并且分子之间通过HONO,NO 2 -HSO 3和NH 3 / H 2形成的弱相互作用O.另外,进行现场观察,并汇总测量数据。相关性分析支持了观察研究期间NO 2向HONO的转化。弱相互作用促进质子转移,导致生成HONO,NO 2 -HSO 3和NH 3 / H 2 O对。































 京公网安备 11010802027423号
京公网安备 11010802027423号