当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron Coordination Polymer, Fe(oxalate)(H2O)2 Nanorods Grown on Nickel Foam via One-Step Electrodeposition as an Efficient Electrocatalyst for Oxygen Evolution Reaction
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-22 , DOI: 10.1021/acs.inorgchem.1c00170 Yang Hai 1 , Li Liu 1 , Yun Gong 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-22 , DOI: 10.1021/acs.inorgchem.1c00170 Yang Hai 1 , Li Liu 1 , Yun Gong 1
Affiliation
|
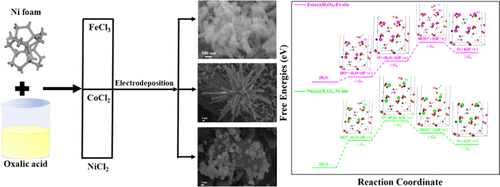
Iron coordination polymer, Fe(ox)(H2O)2 (H2ox = oxalic acid) nanorods were grown on a nickel foam (NF) collector via a one-step electrodeposition method, which can be directly used as a freestanding and binder-free electrode for efficient oxygen evolution reaction (OER) electrocatalysis. The optimum sample, Fe(ox)(H2O)2/NF-(−1.4)-15, was obtained at an electrodeposition potential of −1.4 V vs Ag/AgCl for 15 min, which can deliver OER current densities of 40 and 100 mA cm–2 at overpotentials of 270 and 340 mV, respectively. The sample also exhibits good long-term OER activity during 80 h electrolysis at 20 mA cm–2, which is superior to those of previously reported metal–organic framework (MOF) catalysts. Furthermore, the formation mechanism of the Fe(ox)(H2O)2 nanorods is primarily investigated and the effect of the species of metal ions in MOF on the morphology and OER behavior is also explored. In addition, density functional theory (DFT) calculations reveal that the rate-limiting steps on Fe(ox)(H2O)2 and Ni(ox)(H2O)2 are the formation of Fe*–OOH and Ni*–OH intermediates with maximum ΔG values of 1.628 and 1.710 eV, respectively, which is attributed to the different electronic configurations of Ni and Fe, thus giving rise to different d-band centers and different affinities for adsorbates.
中文翻译:

铁配位聚合物Fe(草酸盐)(H 2 O)2纳米棒通过一步电沉积法在镍泡沫上生长,作为一种高效的氧析出反应电催化剂
铁配位聚合物Fe(ox)(H 2 O)2(H 2 ox =草酸)纳米棒通过一步电沉积法在泡沫镍(NF)收集器上生长,可以直接用作独立式和用于高效氧释放反应(OER)电催化的无粘结剂电极。最佳样品Fe(ox)(H 2 O)2 /NF-(-1.4)-15在相对于Ag / AgCl的电沉积电位为-1.4 V的条件下经过15分钟获得,可以提供40的OER电流密度和100 mA cm –2分别在270和340 mV的过电势下。在20 mA cm –2的电解80 h期间,样品还表现出良好的长期OER活性。优于以前报道的金属-有机骨架(MOF)催化剂。此外,主要研究了Fe(ox)(H 2 O)2纳米棒的形成机理,并研究了MOF中金属离子的种类对形态和OER行为的影响。此外,密度泛函理论(DFT)计算表明,Fe(ox)(H 2 O)2和Ni(ox)(H 2 O)2的限速步骤是Fe * –OOH和Ni *的形成具有最大ΔG的–OH中间体 值分别为1.628和1.710 eV,这归因于Ni和Fe的不同电子构型,因此产生了不同的d带中心和不同的被吸附物亲和力。
更新日期:2021-04-05
中文翻译:

铁配位聚合物Fe(草酸盐)(H 2 O)2纳米棒通过一步电沉积法在镍泡沫上生长,作为一种高效的氧析出反应电催化剂
铁配位聚合物Fe(ox)(H 2 O)2(H 2 ox =草酸)纳米棒通过一步电沉积法在泡沫镍(NF)收集器上生长,可以直接用作独立式和用于高效氧释放反应(OER)电催化的无粘结剂电极。最佳样品Fe(ox)(H 2 O)2 /NF-(-1.4)-15在相对于Ag / AgCl的电沉积电位为-1.4 V的条件下经过15分钟获得,可以提供40的OER电流密度和100 mA cm –2分别在270和340 mV的过电势下。在20 mA cm –2的电解80 h期间,样品还表现出良好的长期OER活性。优于以前报道的金属-有机骨架(MOF)催化剂。此外,主要研究了Fe(ox)(H 2 O)2纳米棒的形成机理,并研究了MOF中金属离子的种类对形态和OER行为的影响。此外,密度泛函理论(DFT)计算表明,Fe(ox)(H 2 O)2和Ni(ox)(H 2 O)2的限速步骤是Fe * –OOH和Ni *的形成具有最大ΔG的–OH中间体 值分别为1.628和1.710 eV,这归因于Ni和Fe的不同电子构型,因此产生了不同的d带中心和不同的被吸附物亲和力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号