当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting charge carrier separation efficiency by constructing an intramolecular DA system towards efficient photoreduction of CO2
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-3-19 , DOI: 10.1039/d0nj05968d Xinyu Zhang 1, 2, 3, 4, 5 , Mei Wang 4, 5, 6, 7, 8 , Xianghai Song 4, 5, 6, 7, 8 , Yongsheng Yan 1, 2, 3, 4, 5 , Pengwei Huo 4, 5, 6, 7, 8 , Yan Yan 4, 5, 6, 7, 8 , Boting Yang 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-3-19 , DOI: 10.1039/d0nj05968d Xinyu Zhang 1, 2, 3, 4, 5 , Mei Wang 4, 5, 6, 7, 8 , Xianghai Song 4, 5, 6, 7, 8 , Yongsheng Yan 1, 2, 3, 4, 5 , Pengwei Huo 4, 5, 6, 7, 8 , Yan Yan 4, 5, 6, 7, 8 , Boting Yang 1, 2, 3, 4
Affiliation
|
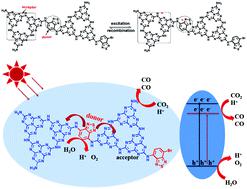
Carbon nitride (CN) has attracted increasing attention in the field of photocatalysis due to its special properties such as visible light response, high stability, and low cost. However, the low separation efficiency of photogenerated electrons and holes limits its catalytic activity. Herein, a novel kind of CN based intramolecular donor–acceptor (DA) system was prepared to facilitate the separation efficiency of photoinduced charge carriers. The catalysts were prepared from CN and 4,7-dibromo-2,1,3-benzothiadiazole (Dbbt) by a simple calcination method. A series of characterization results confirmed that Dbbt is successfully introduced into the framework of CN (CN–Dbbt-x). It was found that the formation of the DA structure leads to the spatial separation of electrons and holes which significantly accelerate the separation efficiency of charge carriers. Moreover, the DA structure played an important role in regulating the bandgap structure, which can increase the optical absorption ability of the catalysts. Also, the DA system led to the formation of a built-in electric field, which remarkably accelerates the migration speed of electrons. CN–Dbbt-0.01 displayed the best photocatalytic CO2 reduction performance, and the CO evolution rate was 4.9 times higher than that of CN. Moreover, the reaction was carried out in water without any sacrificial agents, which makes it a green and environmentally friendly reaction. DFT simulation and experimental results showed that Dbbt and CN respectively serve as the donor and acceptor in CN–Dbbt-0.01.
中文翻译:

通过构建分子内DA系统以实现二氧化碳的有效光还原,提高电荷载流子分离效率
氮化碳(CN)由于其特殊的特性(例如可见光响应,高稳定性和低成本)已经在光催化领域引起了越来越多的关注。然而,光生电子和空穴的低分离效率限制了其催化活性。在此,制备了一种新型的基于CN的分子内供体-受体(DA)系统,以促进光诱导载流子的分离效率。通过简单的煅烧方法,由CN和4,7-二溴-2,1,3-苯并噻二唑(Dbbt)制备催化剂。一系列表征结果证实了Dbbt已成功引入CN(CN–Dbbt- x)。发现DA结构的形成导致电子和空穴的空间分离,这显着加速了电荷载流子的分离效率。而且,DA结构在调节带隙结构中起着重要作用,其可以增加催化剂的光吸收能力。此外,DA系统还导致了内置电场的形成,从而显着加快了电子的迁移速度。CN–Dbbt-0.01显示出最佳的光催化CO 2还原性能,CO释放速率比CN高4.9倍。而且,该反应在没有任何牺牲剂的水中进行,这使其绿色环保。DFT模拟和实验结果表明,Dbbt和CN在CN–Dbbt-0.01中分别充当供体和受体。
更新日期:2021-03-19
中文翻译:

通过构建分子内DA系统以实现二氧化碳的有效光还原,提高电荷载流子分离效率
氮化碳(CN)由于其特殊的特性(例如可见光响应,高稳定性和低成本)已经在光催化领域引起了越来越多的关注。然而,光生电子和空穴的低分离效率限制了其催化活性。在此,制备了一种新型的基于CN的分子内供体-受体(DA)系统,以促进光诱导载流子的分离效率。通过简单的煅烧方法,由CN和4,7-二溴-2,1,3-苯并噻二唑(Dbbt)制备催化剂。一系列表征结果证实了Dbbt已成功引入CN(CN–Dbbt- x)。发现DA结构的形成导致电子和空穴的空间分离,这显着加速了电荷载流子的分离效率。而且,DA结构在调节带隙结构中起着重要作用,其可以增加催化剂的光吸收能力。此外,DA系统还导致了内置电场的形成,从而显着加快了电子的迁移速度。CN–Dbbt-0.01显示出最佳的光催化CO 2还原性能,CO释放速率比CN高4.9倍。而且,该反应在没有任何牺牲剂的水中进行,这使其绿色环保。DFT模拟和实验结果表明,Dbbt和CN在CN–Dbbt-0.01中分别充当供体和受体。































 京公网安备 11010802027423号
京公网安备 11010802027423号