Applied Surface Science ( IF 6.3 ) Pub Date : 2021-03-19 , DOI: 10.1016/j.apsusc.2021.149564 Xuehu Zhong , Junwei Han , Lingling Chen , Wei Liu , Fen Jiao , Hailing Zhu , Wenqing Qin
|
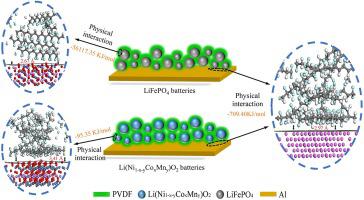
The binding mechanism of polyvinylidene fluoride (PVDF) in lithium ion batteries (LIBs) is important for the development of new binders and the peeling of active materials during the recovery of spent LIBs. This paper focuses on revealing the binding mechanism of PVDF by the simulation calculation using density function theory (DFT) and the analyses of the binding surfaces in LIBs. The results of the process simulation and theoretical calculation indicate that the binding interactions between LiFePO4 (LFP) and PVDF are much stronger than that between PVDF and Al in LFP batteries, whereas, the binding interactions between Li(Ni1-x-yCoxMny)O2 (NCM) and PVDF are weaker than that between PVDF and Al in NCM batteries. Scanning electron microscopy and Auger electron spectroscopy (AES) analyses indicate that PVDF are mainly distributed on the surfaces of LFP in LFP batteries, however, PVDF distribute uniformly between Al and NCM in NCM batteries. In addition, AES analyses indicate that no chemical interaction among the surfaces of active materials, PVDF, and Al, both in LFP and NCM batteries. This study provides a foundation for the development of new binders and the peeling of active materials in LIBs.
中文翻译:

锂离子电池中PVDF的结合机理
锂离子电池(LIB)中聚偏二氟乙烯(PVDF)的结合机理对于开发新的粘合剂以及回收用过的LIB期间活性物质的剥离非常重要。本文着重于通过密度函数理论(DFT)的模拟计算以及对LIBs结合表面的分析来揭示PVDF的结合机理。过程仿真和理论计算的结果表明,在LFP电池中,LiFePO 4(LFP)和PVDF之间的结合作用比PVDF和Al之间的结合作用要强得多,而Li(Ni 1 -xy Co x Mn y)O 2(NCM)和PVDF比NCM电池中的PVDF和Al之间的弱。扫描电子显微镜和俄歇电子能谱(AES)分析表明,PVDF主要分布在LFP电池的LFP表面上,但是PVDF在NCM电池的Al和NCM之间均匀分布。此外,AES分析表明,在LFP和NCM电池中,活性材料,PVDF和Al的表面之间都没有化学相互作用。这项研究为开发新型粘合剂和剥离LIB中的活性物质提供了基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号