当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Antimicrobial Activity Evaluation of Imidazole-Fused Imidazo[2,1-b][1,3,4]thiadiazole Analogues
ChemMedChem ( IF 3.6 ) Pub Date : 2021-03-18 , DOI: 10.1002/cmdc.202100122 Fang Yan Guo 1 , Chang Ji Zheng 2 , Meiyuan Wang 2 , Jiangping Ai 2 , Lan Ying Han 1 , Liu Yang 1 , Ye Fang Lu 1 , Yu Xuan Yang 1 , Ming Guan Piao 2 , Hu-Ri Piao 2 , Chun-Mei Jin 2 , Cheng Hua Jin 1, 2
ChemMedChem ( IF 3.6 ) Pub Date : 2021-03-18 , DOI: 10.1002/cmdc.202100122 Fang Yan Guo 1 , Chang Ji Zheng 2 , Meiyuan Wang 2 , Jiangping Ai 2 , Lan Ying Han 1 , Liu Yang 1 , Ye Fang Lu 1 , Yu Xuan Yang 1 , Ming Guan Piao 2 , Hu-Ri Piao 2 , Chun-Mei Jin 2 , Cheng Hua Jin 1, 2
Affiliation
|
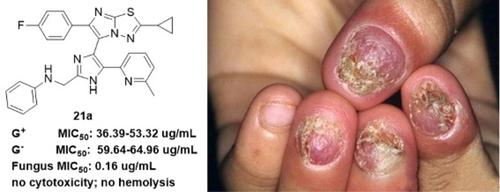
Three series of new imidazole-fused imidazo[2,1-b][1,3,4]thiadiazole analogues (compounds 20 a–g, 21 a–g, and 22 a–g) have been synthesized, and their antibacterial and antifungal activities have been evaluated. All the target compounds showed strong antifungal activity and high selectivity for the test fungus Candida albicans over Gram-positive and -negative bacteria. N-((4-(2-Cyclopropyl-6-(4-fluorophenyl)imidazo[2,1-b][1,3,4]thiadiazol-5-yl)-5-(6-methyl-pyridin-2-yl)-1H-imidazol-2-yl)methyl)aniline (21 a) showed the highest activity against C. albicans (MIC50=0.16 μg/mL), 13 and three times that of the positive control compounds gatifloxacin and fluconazole, respectively. Compounds 21 a and 20 e did not show cytotoxicity against human foreskin fibroblast-1 cells, and compound 21 a was as safe as the positive control compounds in hemolysis tests. These results strongly suggest that some of the compounds produced in this work have value for development as antifungal agents.
中文翻译:

咪唑稠合咪唑[2,1-b][1,3,4]噻二唑类似物的合成及抗菌活性评价
合成了三个系列的新型咪唑稠合咪唑并[2,1- b ][1,3,4]噻二唑类似物(化合物20a - g、21a - g和22a - g),并对其抗菌、已评估抗真菌活性。所有目标化合物均显示出对测试真菌白色念珠菌优于革兰氏阳性和阴性菌的强抗真菌活性和高选择性。N -((4-(2-环丙基-6-(4-氟苯基)咪唑并[2,1- b ][1,3,4]噻二唑-5-基)-5-(6-甲基-吡啶-2) -基)-1 H-咪唑-2-基)甲基)苯胺( 21 a) 对白色念珠菌的活性最高(MIC 50 =0.16 μg/mL),分别是阳性对照化合物加替沙星和氟康唑的 13 倍和 3 倍。化合物21a和20e对人包皮成纤维细胞1没有细胞毒性,在溶血试验中化合物21a与阳性对照化合物一样安全。这些结果有力地表明,在这项工作中产生的一些化合物具有开发作为抗真菌剂的价值。
更新日期:2021-03-18
中文翻译:

咪唑稠合咪唑[2,1-b][1,3,4]噻二唑类似物的合成及抗菌活性评价
合成了三个系列的新型咪唑稠合咪唑并[2,1- b ][1,3,4]噻二唑类似物(化合物20a - g、21a - g和22a - g),并对其抗菌、已评估抗真菌活性。所有目标化合物均显示出对测试真菌白色念珠菌优于革兰氏阳性和阴性菌的强抗真菌活性和高选择性。N -((4-(2-环丙基-6-(4-氟苯基)咪唑并[2,1- b ][1,3,4]噻二唑-5-基)-5-(6-甲基-吡啶-2) -基)-1 H-咪唑-2-基)甲基)苯胺( 21 a) 对白色念珠菌的活性最高(MIC 50 =0.16 μg/mL),分别是阳性对照化合物加替沙星和氟康唑的 13 倍和 3 倍。化合物21a和20e对人包皮成纤维细胞1没有细胞毒性,在溶血试验中化合物21a与阳性对照化合物一样安全。这些结果有力地表明,在这项工作中产生的一些化合物具有开发作为抗真菌剂的价值。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号