当前位置:
X-MOL 学术
›
Adv. Funct. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anchoring Polysulfides and Accelerating Redox Reaction Enabled by Fe-Based Compounds in Lithium–Sulfur Batteries
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-03-18 , DOI: 10.1002/adfm.202100970 Zhensong Qiao 1 , Yinggan Zhang 1 , Zhaohui Meng 2 , Qingshui Xie 1 , Liang Lin 1 , Hongfei Zheng 1 , Baisheng Sa 3 , Jie Lin 1 , Laisen Wang 1 , Dong‐Liang Peng 1
Advanced Functional Materials ( IF 18.5 ) Pub Date : 2021-03-18 , DOI: 10.1002/adfm.202100970 Zhensong Qiao 1 , Yinggan Zhang 1 , Zhaohui Meng 2 , Qingshui Xie 1 , Liang Lin 1 , Hongfei Zheng 1 , Baisheng Sa 3 , Jie Lin 1 , Laisen Wang 1 , Dong‐Liang Peng 1
Affiliation
|
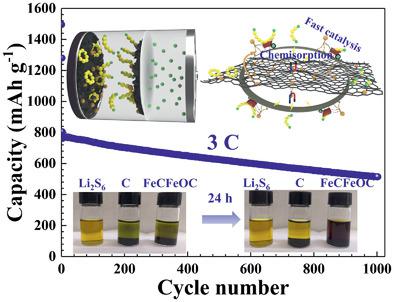
The synergetic mechanism of chemisorption and catalysis play an important role in developing high-performance lithium–sulfur (Li–S) batteries. Herein, a 3D lather-like porous carbon framework containing Fe-based compounds (including Fe3C, Fe3O4, and Fe2O3), named FeCFeOC, is designed as the sulfur host and the interlayer on separator. Due to the strong chemisorption and catalytic ability of FeCFeOC composite, the soluble lithium polysulfides (LiPSs) are first adsorbed and anchored on the surface of the FeCFeOC composite and then are catalyzed to accelerate their conversion reaction. In addition, the FexOy in Fe-based compounds can spontaneously react with LiPSs to form magnetic FeSx species with a larger size, further blocking the penetration of LiPSs cross the separator. As a result, the assembled Li–S cells show excellent long-term stability (748 mAh g−1 over 500 cycles at 1.0 C, and ≈0.036% decay per cycle for 1000 cycles at 3.0 C), a superb rate capability with 659 mAh g−1 at 5.0 C, and lower electrochemical polarization. This work introduces a feasible strategy to anchor and accelerate the conversion of LiPSs by designing the multifunctional Fe-based compounds with high chemisorption and catalytic activity, which advances the large-scale application of high-performance Li–S batteries.
中文翻译:

锚固多硫化物并加速锂硫电池中铁基化合物引起的氧化还原反应
化学吸附和催化的协同机制在开发高性能锂硫(Li–S)电池中起着重要作用。在此,将包含Fe 3 Fe ,Fe 3 O 4和Fe 2 O 3的Fe基化合物(称为FeCFeOC)的3D泡沫状多孔碳骨架设计为硫主体和隔板上的中间层。由于FeCFeOC复合材料的强化学吸附和催化能力,可溶性多硫化锂(LiPSs)首先被吸附并锚定在FeCFeOC复合材料的表面,然后被催化以加速其转化反应。另外,Fe x O y在Fe基化合物中,LiPSs可以自发地与LiPSs反应形成较大尺寸的磁性FeS x物种,从而进一步阻止LiPSs穿过隔膜的渗透。结果,组装好的Li–S电池显示出优异的长期稳定性(1.0 C下500个循环中748 mAh g -1,在3.0 C下1000个循环中每个循环的衰减约为0.036%),具有659的出色速率能力在5.0 C下mAh g -1,并且电化学极化较低。这项工作通过设计具有高化学吸附和催化活性的多功能铁基化合物,引入了一种可行的策略来锚定和加速LiPS的转化,从而推动了高性能Li-S电池的大规模应用。
更新日期:2021-05-25
中文翻译:

锚固多硫化物并加速锂硫电池中铁基化合物引起的氧化还原反应
化学吸附和催化的协同机制在开发高性能锂硫(Li–S)电池中起着重要作用。在此,将包含Fe 3 Fe ,Fe 3 O 4和Fe 2 O 3的Fe基化合物(称为FeCFeOC)的3D泡沫状多孔碳骨架设计为硫主体和隔板上的中间层。由于FeCFeOC复合材料的强化学吸附和催化能力,可溶性多硫化锂(LiPSs)首先被吸附并锚定在FeCFeOC复合材料的表面,然后被催化以加速其转化反应。另外,Fe x O y在Fe基化合物中,LiPSs可以自发地与LiPSs反应形成较大尺寸的磁性FeS x物种,从而进一步阻止LiPSs穿过隔膜的渗透。结果,组装好的Li–S电池显示出优异的长期稳定性(1.0 C下500个循环中748 mAh g -1,在3.0 C下1000个循环中每个循环的衰减约为0.036%),具有659的出色速率能力在5.0 C下mAh g -1,并且电化学极化较低。这项工作通过设计具有高化学吸附和催化活性的多功能铁基化合物,引入了一种可行的策略来锚定和加速LiPS的转化,从而推动了高性能Li-S电池的大规模应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号