当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bioinspired Diversification Approach Toward the Total Synthesis of Lycodine-Type Alkaloids
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-03-17 , DOI: 10.1021/jacs.1c00457
Hannah M S Haley 1 , Stefan E Payer 1 , Sven M Papidocha 1 , Simon Clemens 1 , Jonathan Nyenhuis 1 , Richmond Sarpong 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-03-17 , DOI: 10.1021/jacs.1c00457
Hannah M S Haley 1 , Stefan E Payer 1 , Sven M Papidocha 1 , Simon Clemens 1 , Jonathan Nyenhuis 1 , Richmond Sarpong 1
Affiliation
|
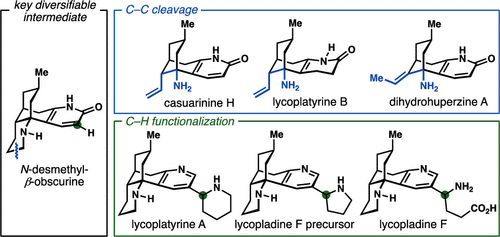
Nitrogen heterocycles (azacycles) are common structural motifs in numerous pharmaceuticals, agrochemicals, and natural products. Many powerful methods have been developed and continue to be advanced for the selective installation and modification of nitrogen heterocycles through C–H functionalization and C–C cleavage approaches, revealing new strategies for the synthesis of targets containing these structural entities. Here, we report the first total syntheses of the lycodine-type Lycopodium alkaloids casuarinine H, lycoplatyrine B, lycoplatyrine A, and lycopladine F as well as the total synthesis of 8,15-dihydrohuperzine A through bioinspired late-stage diversification of a readily accessible common precursor, N-desmethyl-β-obscurine. Key steps in the syntheses include oxidative C–C bond cleavage of a piperidine ring in the core structure of the obscurine intermediate and site-selective C–H borylation of a pyridine nucleus to enable cross-coupling reactions.
中文翻译:

莱可定型生物碱全合成的仿生多样化方法
氮杂环(氮杂环)是许多药物、农用化学品和天然产物中常见的结构基序。通过C-H官能化和C-C裂解方法选择性安装和修饰氮杂环的许多强大方法已经被开发出来并继续得到发展,揭示了包含这些结构实体的靶标合成的新策略。在这里,我们报告了石松碱型石松生物碱木麻黄碱 H、石松碱 B、石松碱 A 和石松碱 F 的首次全合成,以及通过易于获得的生物启发后期多样化来全合成 8,15-二氢石杉碱 A。常见前体, N-去甲基-β-暗色碱。合成中的关键步骤包括暗色中间体核心结构中哌啶环的氧化C-C键断裂以及吡啶核的位点选择性C-H硼基化以实现交叉偶联反应。
更新日期:2021-03-31
中文翻译:

莱可定型生物碱全合成的仿生多样化方法
氮杂环(氮杂环)是许多药物、农用化学品和天然产物中常见的结构基序。通过C-H官能化和C-C裂解方法选择性安装和修饰氮杂环的许多强大方法已经被开发出来并继续得到发展,揭示了包含这些结构实体的靶标合成的新策略。在这里,我们报告了石松碱型石松生物碱木麻黄碱 H、石松碱 B、石松碱 A 和石松碱 F 的首次全合成,以及通过易于获得的生物启发后期多样化来全合成 8,15-二氢石杉碱 A。常见前体, N-去甲基-β-暗色碱。合成中的关键步骤包括暗色中间体核心结构中哌啶环的氧化C-C键断裂以及吡啶核的位点选择性C-H硼基化以实现交叉偶联反应。

































 京公网安备 11010802027423号
京公网安备 11010802027423号