当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel Sesquiterpenoid Skeletons by Wagner–Meerwein Rearrangements of Longipinane-9,13-diol-1-one Derivatives
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-03-18 , DOI: 10.1021/acs.jnatprod.0c01160 Cecilia Ruiz-Ferrer 1 , Luisa U Román-Marín 1 , Juan D Hernández-Hernández 1 , Carlos M Cerda-García-Rojas 2 , Pedro Joseph-Nathan 2
Journal of Natural Products ( IF 3.3 ) Pub Date : 2021-03-18 , DOI: 10.1021/acs.jnatprod.0c01160 Cecilia Ruiz-Ferrer 1 , Luisa U Román-Marín 1 , Juan D Hernández-Hernández 1 , Carlos M Cerda-García-Rojas 2 , Pedro Joseph-Nathan 2
Affiliation
|
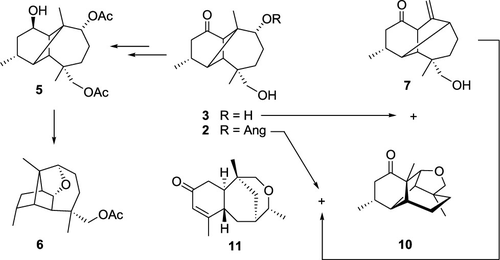
The partial or total hydrolysis of (3R,4S,5S,6S,9R,10R,11R)-9,13-diangeloyloxylongipinan-1-one (1), isolated from the roots of Stevia viscida, gave alcohols 2 or 3, respectively, which were subjected to molecular rearrangements with boron trifluoride etherate. Compound 2 afforded (3R,4R,5R,6S,9R,10S,11S)-11,13-oxyneomorelian-1-one (10) and (4S,5R,6S,8S,10R)-10,13-oxyneojiquilp-2-en-1-one (11), both possessing novel sesquiterpenoid skeletons. In turn, 3 provided (3R,4R,5S,6S,9R,11R)-13-hydroxymoreli-10(14)-en-1-one (7) and 10. Acetylation of 3 gave 4, thus allowing reduction of the C-1 carbonyl group to yield 5, which was rearranged to (1S,3R,4S,5S,6S,9R,10R,11R)-13-acetoxy-9,11-epoxyjiquilpane (6), while an attempt to mesylate 3 directly gave rearranged (3R,4R,5S,6S,9R,11R)-13-mesyloxymoreli-10(14)-en-1-one (8) through expulsion of the C-9 mesylate group by the antiperiplanar C-4–C-10 bond migration to C-4–C-9. In addition, treatment of 1 with boron trifluoride etherate generated (3R,4R,5S,6S,9R,11R)-13-angeloyloxymoreli-10(14)-en-1-one (9). The structures of 2–11 were elucidated by 1D and 2D NMR experiments and those of 2, 3, 8, 10, and 11 were confirmed by single-crystal X-ray diffraction analysis.
中文翻译:

由Wagner-Meerwein 对Longipinane-9,13-diol-1-one 衍生物进行重排的新型倍半萜类骨架
(3 R ,4 S ,5 S ,6 S ,9 R ,10 R ,11 R )-9,13-diangeloyloxylongipinan-1-one ( 1 )的部分或全部水解,从甜叶菊的根中分离出来,分别得到醇2或3,将其与三氟化硼醚合物进行分子重排。化合物2得到 (3 R ,4 R ,5 R ,6 S ,9 R ,10 S ,11 S )-11,13-oxyneomorelian-1-one ( 10) 和 (4 S ,5 R ,6 S ,8 S ,10 R )-10,13-oxyneojiquilp-2-en-1-one ( 11 ),两者都具有新型倍半萜类骨架。反过来,3提供了 (3 R ,4 R ,5 S ,6 S ,9 R ,11 R )-13-hydroxymoreli-10(14)-en-1-one ( 7 ) 和10。3乙酰化得到4,从而使 C-1 羰基还原得到5,其重排为 (1 S ,3 R ,4S ,5 S ,6 S ,9 R ,10 R ,11 R )-13-乙酰氧基-9,11-epoxyjiquilpane ( 6 ),而试图将3直接甲磺酸化得到重排 (3 R ,4 R ,5 S , 6 S ,9 R ,11 R )-13-mesyloxymoreli-10(14)-en-1-one ( 8 ) 通过反周向 C-4–C-10 键迁移到 C -4–C-9。此外,用三氟化硼醚合物处理1生成 (3 R ,4 R ,5 S ,6S ,9 R ,11 R )-13-angeloyloxymoreli-10(14)-en-1-one ( 9 )。的结构2 - 11通过一维和二维NMR实验阐明和那些的2,3,8,10,和11通过单晶X射线衍射分析得到了证实。
更新日期:2021-04-23
中文翻译:

由Wagner-Meerwein 对Longipinane-9,13-diol-1-one 衍生物进行重排的新型倍半萜类骨架
(3 R ,4 S ,5 S ,6 S ,9 R ,10 R ,11 R )-9,13-diangeloyloxylongipinan-1-one ( 1 )的部分或全部水解,从甜叶菊的根中分离出来,分别得到醇2或3,将其与三氟化硼醚合物进行分子重排。化合物2得到 (3 R ,4 R ,5 R ,6 S ,9 R ,10 S ,11 S )-11,13-oxyneomorelian-1-one ( 10) 和 (4 S ,5 R ,6 S ,8 S ,10 R )-10,13-oxyneojiquilp-2-en-1-one ( 11 ),两者都具有新型倍半萜类骨架。反过来,3提供了 (3 R ,4 R ,5 S ,6 S ,9 R ,11 R )-13-hydroxymoreli-10(14)-en-1-one ( 7 ) 和10。3乙酰化得到4,从而使 C-1 羰基还原得到5,其重排为 (1 S ,3 R ,4S ,5 S ,6 S ,9 R ,10 R ,11 R )-13-乙酰氧基-9,11-epoxyjiquilpane ( 6 ),而试图将3直接甲磺酸化得到重排 (3 R ,4 R ,5 S , 6 S ,9 R ,11 R )-13-mesyloxymoreli-10(14)-en-1-one ( 8 ) 通过反周向 C-4–C-10 键迁移到 C -4–C-9。此外,用三氟化硼醚合物处理1生成 (3 R ,4 R ,5 S ,6S ,9 R ,11 R )-13-angeloyloxymoreli-10(14)-en-1-one ( 9 )。的结构2 - 11通过一维和二维NMR实验阐明和那些的2,3,8,10,和11通过单晶X射线衍射分析得到了证实。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号