当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Dechlorination of Dichloromethane in Water Using a Heterogenized Molecular Copper Complex
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-18 , DOI: 10.1021/acs.inorgchem.0c03833 Caroline K. Williams 1 , Gavin A. McCarver 2 , Amir Lashgari 1 , Konstantinos D. Vogiatzis 2 , Jianbing “Jimmy” Jiang 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-18 , DOI: 10.1021/acs.inorgchem.0c03833 Caroline K. Williams 1 , Gavin A. McCarver 2 , Amir Lashgari 1 , Konstantinos D. Vogiatzis 2 , Jianbing “Jimmy” Jiang 1
Affiliation
|
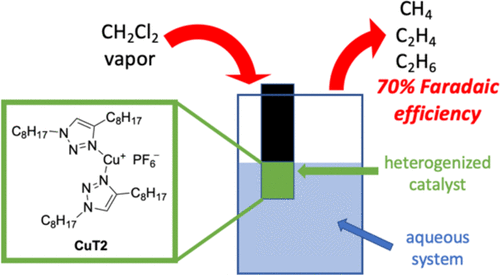
The remediation of organohalides from water is a challenging process in environment protection and water treatment. Herein, we report a molecular copper(I) complex with two triazole units, CuT2, in a heterogeneous aqueous system that is capable of dechlorinating dichloromethane (CH2Cl2) to afford hydrocarbons (methane, ethane, and ethylene). The catalytic performance is evaluated in water and presented high Faradaic efficiency (average 70% CH4) across a range of potentials (−1.1 to −1.6 V vs Ag/AgCl) and high activity (maximum −25.1 mA/cm2 at −1.6 V vs Ag/AgCl) with a turnover number of 2.0 × 107. The CuT2 catalyst also showed excellent stability for 14 h of constant exposure to CH2Cl2 and 10 h of CH2Cl2 exposure cycling. The control compound, a copper-free triazole unit (T1), was also investigated under the same condition and showed inferior catalytic activity, indicating the importance of the copper center. Plausible catalytic mechanisms are proposed for the formation of C1 and C2 products via radical intermediates. Computational studies provided additional insight into the reaction mechanism and the selectivity toward the CH4 formation. The findings in this study demonstrate that complex CuT2 is an efficient and stable catalyst for the dehalogenation of CH2Cl2 and could potentially be used for the exploration of the removal of halogenated species from aqueous systems.
中文翻译:

非均相分子铜配合物对水中二氯甲烷的电催化脱氯
从水中修复有机卤化物在环境保护和水处理中是一个具有挑战性的过程。在本文中,我们报告了一种分子铜(I)配合物,该分子具有两个三唑单元CuT2,处于非均相的水性体系中,该体系能够对二氯甲烷(CH 2 Cl 2)进行脱氯,从而提供碳氢化合物(甲烷,乙烷和乙烯)。在水中评估了催化性能,并在一定范围的电势(相对于Ag / AgCl相对于-1.1至-1.6 V)中表现出高的法拉第效率(平均70%CH 4)和高活性(在-1.6时具有最大-25.1 mA / cm 2) V vs Ag / AgCl),周转数为2.0×10 7。该CUT2催化剂在持续暴露于CH 2 Cl 2 14小时和CH 2 Cl 2暴露循环10 h中也表现出出色的稳定性。在相同条件下还研究了对照化合物,即无铜三唑单元(T1),并且显示出较低的催化活性,表明了铜中心的重要性。提出了通过自由基中间体形成C 1和C 2产物的可能的催化机理。计算研究为反应机理和对CH 4形成的选择性提供了更多的见解。这项研究的结果表明,复杂的CuT2是用于CH 2 Cl 2脱卤的有效和稳定的催化剂,可潜在地用于探索从水性体系中除去卤代物质的方法。
更新日期:2021-04-05
中文翻译:

非均相分子铜配合物对水中二氯甲烷的电催化脱氯
从水中修复有机卤化物在环境保护和水处理中是一个具有挑战性的过程。在本文中,我们报告了一种分子铜(I)配合物,该分子具有两个三唑单元CuT2,处于非均相的水性体系中,该体系能够对二氯甲烷(CH 2 Cl 2)进行脱氯,从而提供碳氢化合物(甲烷,乙烷和乙烯)。在水中评估了催化性能,并在一定范围的电势(相对于Ag / AgCl相对于-1.1至-1.6 V)中表现出高的法拉第效率(平均70%CH 4)和高活性(在-1.6时具有最大-25.1 mA / cm 2) V vs Ag / AgCl),周转数为2.0×10 7。该CUT2催化剂在持续暴露于CH 2 Cl 2 14小时和CH 2 Cl 2暴露循环10 h中也表现出出色的稳定性。在相同条件下还研究了对照化合物,即无铜三唑单元(T1),并且显示出较低的催化活性,表明了铜中心的重要性。提出了通过自由基中间体形成C 1和C 2产物的可能的催化机理。计算研究为反应机理和对CH 4形成的选择性提供了更多的见解。这项研究的结果表明,复杂的CuT2是用于CH 2 Cl 2脱卤的有效和稳定的催化剂,可潜在地用于探索从水性体系中除去卤代物质的方法。















































 京公网安备 11010802027423号
京公网安备 11010802027423号