Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2021-03-17 , DOI: 10.1016/j.bmcl.2021.127963 Juyoung Jung 1 , Hongchul Yoon 2 , Te-Ik Sohn 2 , Kyusic Jang 2 , Yeongran Yoo 2 , Ilji Jeong 2 , Jae Eui Shin 2 , Jin Hee Lee 2 , Jihyae Ann 3 , Jeewoo Lee 3
|
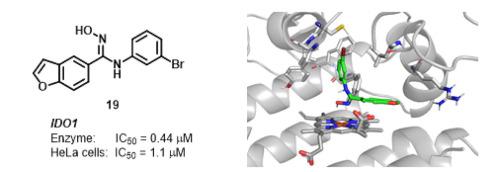
Human indoleamine 2,3-dioxygenase 1 (hIDO1) and tryptophan dioxygenase (hTDO) are rate-limiting enzymes in the kynurenine pathway (KP) of L-tryptophan (L-Trp) metabolism and are becoming key drug targets in the combination therapy of checkpoint inhibitors in immunoncology. To discover a selective and potent IDO1 inhibitor, a structure-activity relationship (SAR) study of N-hydroxybenzofuran-5-carboximidamide as a novel scaffold was investigated in a systematic manner. Among the synthesized compounds, the N-3-bromophenyl derivative 19 showed the most potent inhibition, with an IC50 value of 0.44 μM for the enzyme and 1.1 μM in HeLa cells. The molecular modeling of 19 with the X-ray crystal structure of IDO1 indicated that dipole-ionic interactions with heme iron, halogen bonding with Cys129 and the two hydrophobic interactions were important for the high potency of 19.
中文翻译:

发现 5-(N-Hydroxycarbamimidoyl) 苯并呋喃衍生物作为新型吲哚胺 2,3-双加氧酶 1 (IDO1) 抑制剂
人吲哚胺 2,3-双加氧酶 1 ( h IDO1) 和色氨酸双加氧酶 ( h TDO) 是L-色氨酸 ( L- Trp) 代谢犬尿氨酸途径 (KP) 的限速酶,正在成为组合中的关键药物靶点免疫肿瘤学中检查点抑制剂的治疗。为了发现一种选择性和有效的 IDO1 抑制剂,以系统的方式研究了N-羟基苯并呋喃-5-羧酰亚胺酰胺作为一种新型支架的构效关系 (SAR) 研究。在合成的化合物中,N -3-溴苯基衍生物 19 显示出最有效的抑制作用,IC 50该酶的值为 0.44 μM,HeLa 细胞中的值为 1.1 μM。具有 IDO1 的 X 射线晶体结构的 19 的分子模型表明,与血红素铁的偶极离子相互作用、与 Cys129 的卤素键合以及两种疏水相互作用对于 19 的高效能很重要。






























 京公网安备 11010802027423号
京公网安备 11010802027423号