当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereoselective Synthesis of 1,2-Dihydrobenzofuro[3,2-b]pyridines via a Carbon–Carbon Double-Bond Cleavage/Rearrangement Cascade
Organic Letters ( IF 4.9 ) Pub Date : 2021-03-17 , DOI: 10.1021/acs.orglett.1c00564 Chi-Fan Zhu 1 , Ling-Qi Chen 1 , Wen-Juan Hao 1 , Chen-Chang Cui 1 , Shu-Jiang Tu 1 , Bo Jiang 1
Organic Letters ( IF 4.9 ) Pub Date : 2021-03-17 , DOI: 10.1021/acs.orglett.1c00564 Chi-Fan Zhu 1 , Ling-Qi Chen 1 , Wen-Juan Hao 1 , Chen-Chang Cui 1 , Shu-Jiang Tu 1 , Bo Jiang 1
Affiliation
|
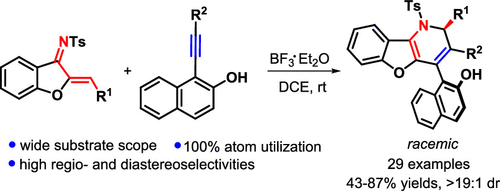
A new Lewis acid-catalyzed [2 + 2] cycloaddition/retroelectrocyclization (CA-RE)/1,6-addition relay of aurone-derived 1-azadienes and 1-alkynylnaphthalen-2-ols has been reported, leading to the regio- and diastereoselective synthesis of 1,2-dihydrobenzofuro[3,2-b]pyridine with a chiral carbon center and an axial chirality in good yields. This protocol enables the C–C double-bond scission/recombination to rapidly construct aza-heterocyclic architectures and features 100% atom utilization, a wide substrate scope, good compatibility with substituents, and excellent diastereoselectivity.
中文翻译:

通过碳-碳双键裂解/重排的级联非对映选择性合成1,2-二氢苯并呋喃[3,2- b ]吡啶
据报道,一种新的路易斯酸催化的[2 + 2]环化/逆电环化(CA-RE)/ 1,6-加成反应的是由金酮衍生的1-氮杂二烯和1-炔基萘-2-醇,导致区域-且具有良好收率的具有手性碳中心和轴向手性的1,2-二氢苯并呋喃并[3,2- b ]吡啶的非对映选择性合成。该协议使CC双键断裂/重组能够快速构建氮杂杂环结构,并具有100%的原子利用率,广泛的底物范围,与取代基的良好相容性以及出色的非对映选择性。
更新日期:2021-04-02
中文翻译:

通过碳-碳双键裂解/重排的级联非对映选择性合成1,2-二氢苯并呋喃[3,2- b ]吡啶
据报道,一种新的路易斯酸催化的[2 + 2]环化/逆电环化(CA-RE)/ 1,6-加成反应的是由金酮衍生的1-氮杂二烯和1-炔基萘-2-醇,导致区域-且具有良好收率的具有手性碳中心和轴向手性的1,2-二氢苯并呋喃并[3,2- b ]吡啶的非对映选择性合成。该协议使CC双键断裂/重组能够快速构建氮杂杂环结构,并具有100%的原子利用率,广泛的底物范围,与取代基的良好相容性以及出色的非对映选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号