Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2020-11-04 , DOI: 10.1016/j.cclet.2020.11.005
Penglong Wang , Qin Zhu , Yi Wang , Guixiang Zeng , Jun Zhu , Congqing Zhu
|
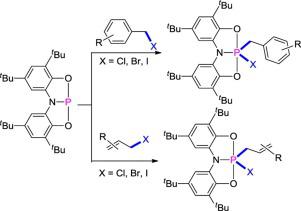
The σ-bond activation by main group element has received enormous attention from theoretical and experimental chemists. Here, the reaction of C–X (X = Cl, Br, I) bonds in benzyl and allyl halides with a pincer-type phosphorus(III) species was reported. A series of structurally robust phosphorus(V) compounds were formed via the formal oxidative addition reactions of C–X bonds to the phosphorus(III) center. Density functional theory calculations show that the nucleophilic addition process is more favorable than the direct oxidative addition mechanism. Isomerization of bent structures of phosphorus(III) compound to poorly nucleophilic compounds to undergo further C–X bond activation can be rationalized by frontier molecule orbital analysis. This study not only provides a deep understanding of the reactivity of phosphorus(III) species but also demonstrates a potential of main group elements for the small-molecule activation.
中文翻译:

通过结构受限的磷(III)平台激活碳-卤素键
通过主族元素进行的σ键活化受到了理论和实验化学家的极大关注。在这里,有报道称苄基和烯丙基卤化物中的C–X(X = Cl,Br,I)键与钳型磷(III)的反应。通过以下方法形成了一系列结构坚固的磷(V)化合物C–X键与磷(III)中心的正式氧化加成反应。密度泛函理论计算表明,亲核加成过程比直接氧化加成机理更有利。磷(III)化合物的弯曲结构异构化为亲核性较差的化合物,以进行进一步的C–X键活化作用,这可以通过前沿分子轨道分析来合理化。这项研究不仅提供了对磷(III)物种的反应性的深刻理解,而且还展示了主要基团元素对小分子活化的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号