Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2020-11-10 , DOI: 10.1016/j.cclet.2020.11.021 Haichao Zhu , Meihua Liu , Haiyan Li , Ting Guan , Qi Zhang , Yang Chen , Yingxiang Liu , Rolf R. Hartmann , Lina Yin , Qingzhong Hu
|
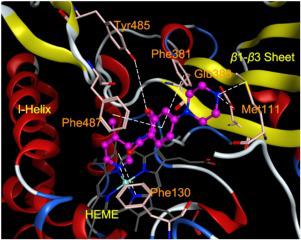
Exorbitant aldosterone is closely associated with various severe diseases, including congestive heart failure and chronic kidney disease. As aldosterone synthase is the pivotal enzyme in aldosterone biosynthesis, its inhibition constitutes a promising treatment for these diseases. Via a structure-based approach, a series of pyridyl substituted 3,4-dihydrobenzo[f][1,4]oxazepin-5(2H)-ones were designed as inhibitors of aldosterone synthase. Six compounds (5j, 5l, 5m 5w, 5x and 5y) distinguished themselves with potent inhibition (IC50 <100 nmol/L) and high selectivity over homogenous 11β-hydroxylase. As the most promising compound, 5x exhibited an IC50 of 12 nmol/L and an excellent selectivity factor (SF) of 157, which are both superior to those of the reference fadrazole (IC50 = 21 nmol/L, SF = 7). Importantly, 5x showed no inhibition against steroidogenic CYP17, CYP19 and a panel of hepatic CYP enzymes indicating an outstanding safety profile. As it manifested satisfactory pharmacokinetic properties in rats, compound 5x was considered as a drug candidate for further development.
中文翻译:

吡啶基取代的苯并恶氮酮作为醛固酮合酶的有效和选择性抑制剂的设计、合成和生物学评价
过高的醛固酮与各种严重疾病密切相关,包括充血性心力衰竭和慢性肾病。由于醛固酮合酶是醛固酮生物合成的关键酶,因此抑制它是治疗这些疾病的一种有希望的方法。通过基于结构的方法,一系列吡啶基取代的 3,4-二氢苯并[ f ][1,4] oxazepin-5(2 H )-ones 被设计为醛固酮合酶的抑制剂。六种化合物(5j、5l、5m 5w、5x和5y)具有显着的抑制作用(IC 50 <100 nmol/L)和对同质 11 β 的高选择性。-羟化酶。作为最有前景的化合物,5x的 IC 50为 12 nmol/L,选择性因子 (SF) 为 157,均优于参考法德拉唑 (IC 50 = 21 nmol/L, SF = 7) . 重要的是,5x对类固醇生成的 CYP17、CYP19 和一组肝 CYP 酶没有抑制作用,表明其具有出色的安全性。由于它在大鼠中表现出令人满意的药代动力学特性,因此化合物5x被认为是进一步开发的候选药物。































 京公网安备 11010802027423号
京公网安备 11010802027423号