Journal of Power Sources ( IF 8.1 ) Pub Date : 2021-03-16 , DOI: 10.1016/j.jpowsour.2021.229760 Hailemariam Kassa Bezabh , Shuo-Feng Chiu , Teklay Mezgebe Hagos , Meng-Che Tsai , Yosef Nikodimos , Haylay Ghidey Redda , Wei-Nien Su , Bing Joe Hwang
|
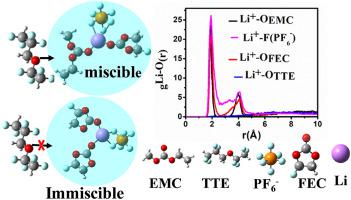
Developing electrolytes with a higher oxidation potential is essential to the performance of lithium-ion batteries (LIBs). Fluorine-containing solvent (1, 1, 2, 2-tetrafluoroethyl-2, 2, 3, 3-tetrafluoropropyl ether (TTE)) is a promising candidate for high-voltage use; however, its weak interaction with lithium hexafluorophosphate (LiPF6) containing electrolyte results in phase separation and causes concerns in practical applications. In this work, ethyl methyl carbonate (EMC) is selected to resolve the phase separation. The solvation structure, oxidation stability, and transport property of 1 M LiPF6 in fluoroethylene carbonate (FEC)/TTE and FEC/TTE/EMC (3:7 and 3:5:2 by vol., respectively) are investigated. The solvation energy of TTE in LiPF6 can be greatly increased by the inclusion of EMC and achieve improved thermodynamic stability. Upon adding EMC, the solvation structure is altered from Li+(FEC)2(PF6−) to Li+(FEC)(EMC)(PF6−) and confirmed by the Raman spectroscopy result. The transference number of Li+ in the improved electrolyte also evidently increases, as confirmed both theoretically and experimentally. Besides, 1 M LiPF6 in EFC/TTE/EMC (3:5: 2 by vol.) exhibits high oxidation potential ~ 5.31 V (vs. Li/Li+) and significantly enhances the transference number of Li+. This work offers a fluorine-containing electrolyte with a stable phase and high oxidation potential for LIB's practical application.
中文翻译:

碳酸乙基甲基酯在氟化电解质中对锂离子电池离子迁移和相稳定性的桥接作用
开发具有更高氧化电位的电解质对于锂离子电池(LIB)的性能至关重要。含氟溶剂(1,1,2,2-四氟乙基-2,2,3,3-四氟丙基醚(TTE))是用于高压用途的有前途的候选材料。然而,其与含电解质的六氟磷酸锂(LiPF 6)的弱相互作用导致相分离并引起实际应用的关注。在这项工作中,选择碳酸乙基甲基酯(EMC)来解决相分离。研究了1 M LiPF 6在碳酸氟代亚乙酯(FEC)/ TTE和FEC / TTE / EMC(分别为3:7和3:5:2,按体积计)中的溶剂化结构,氧化稳定性和传输性能。LiPF 6中TTE的溶剂化能通过包含EMC可以大大提高性能,并提高热力学稳定性。在加入EMC,溶剂化结构选自Li改变+(FEC)2(PF 6 - )对于Li +(FEC)(EMC)(PF 6 - )和由拉曼光谱的结果证实。理论上和实验上都证实,改进的电解质中Li +的转移数也明显增加。此外,EFC / TTE / EMC中的1 M LiPF 6(按体积计3:5:2)显示出〜5.31 V(vs。Li / Li +)的高氧化电位,并显着提高了Li +的转移数。这项工作为LIB的实际应用提供了一种具有稳定相和高氧化电位的含氟电解质。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号