当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Isobaric Vapor–Liquid Equilibria for Binary Mixtures of 2-Phenylethanol with 1-Phenylethanol, 2-Phenylpropan-2-ol, Ethylbenzene, and Isopropylbenzene at 101.3 kPa
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-16 , DOI: 10.1021/acs.jced.0c01040 Sudip Das , Shashwata Ghosh , Mohd Nadeem , Srinivas Seethamraju , Sanjay Mahajani
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2021-03-16 , DOI: 10.1021/acs.jced.0c01040 Sudip Das , Shashwata Ghosh , Mohd Nadeem , Srinivas Seethamraju , Sanjay Mahajani
|
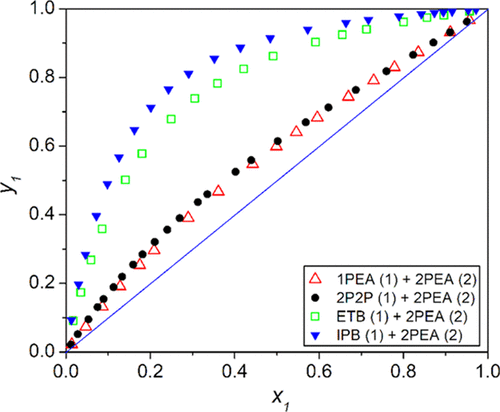
The isobaric binary vapor–liquid equilibrium (VLE) data for the mixture of 2-phenylethanol (2PEA) with 1-phenylethanol (1PEA), 2-phenylpropan-2-ol (2P2P), ethylbenzene (ETB), and isopropylbenzene (IPB) is generated at 101.3 kPa. The Fredenslund test and Redlich–Kister area test methods were used to confirm the consistency of the measured VLE experimental data. Subsequently, the data sets are correlated by the nonrandom two-liquid (NRTL) and universal quasi-chemical (UNIQUAC) thermodynamic models, and the binary interaction parameters for the two models were estimated using the Britt–Luecke algorithm and the maximum likelihood objective function. The estimation was found to be good for both the activity coefficient models.
中文翻译:

2-苯基乙醇与1-苯基乙醇,2-苯基丙烷-2-醇,乙苯和异丙苯的二元混合物的等压蒸气-液体平衡在101.3 kPa
2-苯基乙醇(2PEA)与1-苯基乙醇(1PEA),2-苯基丙烷-2-醇(2P2P),乙苯(ETB)和异丙苯(IPB)的混合物的等压二元气液平衡(VLE)数据产生的压力为101.3 kPa。Fredenslund检验和Redlich-Kister面积检验方法用于确认所测得的VLE实验数据的一致性。随后,数据集通过非随机两液(NRTL)和通用拟化学(UNIQUAC)热力学模型进行关联,并使用Britt-Luecke算法和最大似然目标函数估算了这两个模型的二元相互作用参数。发现该估计对于两个活度系数模型均是良好的。
更新日期:2021-04-08
中文翻译:

2-苯基乙醇与1-苯基乙醇,2-苯基丙烷-2-醇,乙苯和异丙苯的二元混合物的等压蒸气-液体平衡在101.3 kPa
2-苯基乙醇(2PEA)与1-苯基乙醇(1PEA),2-苯基丙烷-2-醇(2P2P),乙苯(ETB)和异丙苯(IPB)的混合物的等压二元气液平衡(VLE)数据产生的压力为101.3 kPa。Fredenslund检验和Redlich-Kister面积检验方法用于确认所测得的VLE实验数据的一致性。随后,数据集通过非随机两液(NRTL)和通用拟化学(UNIQUAC)热力学模型进行关联,并使用Britt-Luecke算法和最大似然目标函数估算了这两个模型的二元相互作用参数。发现该估计对于两个活度系数模型均是良好的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号