当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anion-Recognition Studies of a Rhenium(I) 4-Mercaptopyridine Compound and Its Ligand-Coupling Products
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-15 , DOI: 10.1021/acs.inorgchem.0c03723 Biing-Chiau Tzeng, I-Lin Lin, Wen-Hui Chen, Gene-Hsiang Lee
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2021-03-15 , DOI: 10.1021/acs.inorgchem.0c03723 Biing-Chiau Tzeng, I-Lin Lin, Wen-Hui Chen, Gene-Hsiang Lee
|
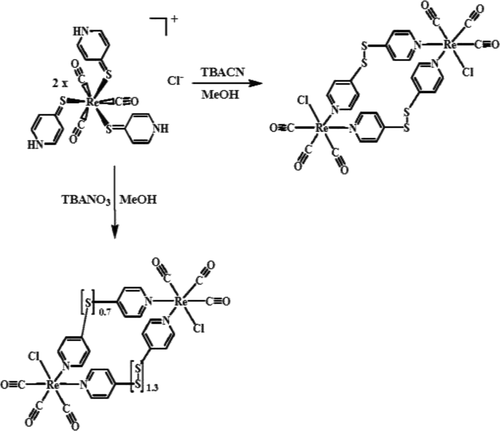
The reaction of Re(CO)5Cl with 4-mercaptopyridine (4-PySH) led to the formation of [Re(CO)3(4-HPyS)3]Cl (1), showing three hydrogen-bonding donors of 4-PySH ligands as well as a characteristic ligand-to-metal charge-transfer absorption at ca. 380 nm. In this regard, a variety of anions, i.e., CN–, OAc–, F–, Cl–, Br–, I–, PF6–, NO3–, ClO4–, and H2PO4–, were examined to study anion-recognition studies through hydrogen-bonding functionalities. Upon the addition of CN– to a methanolic solution of complex 1, a remarkable spectral change with an isosbestic point at ca. 314 nm in the absorption spectra was observed, with a binding constant (Kb) calculated to be 24770 M–1. Moreover, the OAc– anion also shows a similar trend, but a mild spectral change, with Kb calculated to be 2170 M–1. Unlike those of CN– and OAc–, the addition of F–, Cl–, Br–, and I– anions causes a less pronounced spectral change with an isosbestic point at ca. 350 nm and Kb calculated to be 2863–750 M–1. However, almost no spectral change can be observed for other anions (i.e., PF6–, NO3–, H2PO4–, and ClO4–). Interestingly, the molecular loops of [Re(CO)3Cl(Py2S2)]2 (2; Py2S2 = 4,4′-dipyridyl disulfide) and [Re(CO)3Cl(Py2S)0.35(Py2S2)0.65]2 (3; Py2S = 4,4′-dipyridyl sulfide) can be isolated and structurally characterized by X-ray diffraction, where those crystals were grown from diethyl ether diffusion into a methanolic solution of complex 1 with [Bu4N]CN and [Bu4N]NO3, respectively. It is noted that such unusual ligand-coupling reactions toward the homoligand and hybrid-ligand loops of complexes 2 and 3 can be achieved at room temperature in this study.
中文翻译:

hen(I)4-巯基吡啶化合物及其配体偶联产物的阴离子识别研究
Re(CO)5 Cl与4-巯基吡啶(4-PySH)的反应导致形成[Re(CO)3(4-HPyS)3 ] Cl(1),显示出三个氢键供体的4- PySH配体以及特征性配体到金属的电荷转移吸收约为。380海里 在这一点上,各种阴离子的,即,CN -,OAC -,F - ,氯- ,溴- ,我-,PF 6 -,NO 3 -,CLO 4 - ,和H 2 PO 4 -进行了研究,以通过氢键功能研究阴离子识别研究。在络合物1的甲醇溶液中添加CN –后,光谱的显着变化,等渗点约为ca。在吸收光谱中观察到314 nm,结合常数(K b)计算为24770 M –1。此外,OAC -阴离子也显示了类似的趋势,但有轻度的光谱变化,ķ b计算为2170中号-1。不像那些CN的-和OAC - ,增加的F - ,氯- ,溴-和我–阴离子引起不太明显的光谱变化,等渗点为ca。350 nm和K b计算为2863–750 M –1。然而,几乎没有光谱变化可以为其他阴离子观察(即,PF 6 -,NO 3 -,H 2 PO 4 - ,和C10 4 - )。有趣的是,[Re(CO)3 Cl(Py 2 S 2)] 2(2 ; Py 2 S 2 = 4,4'-二吡啶基二硫化物)和[Re(CO)3 Cl(Py 2 S)0.35(Py 2 S 2)0.65 ] 2(3 ; Py 2 S = 4,4'-二吡啶硫醚)可以通过X射线衍射分离和结构表征,其中那些晶体是从乙醚扩散到甲醇溶液中生长的分别具有[Bu 4 N] CN和[Bu 4 N] NO 3的配合物1的结构。注意,在这项研究中,可以在室温下实现对配合物2和3的同配体和杂配体环的这种不寻常的配体偶联反应。
更新日期:2021-04-05
中文翻译:

hen(I)4-巯基吡啶化合物及其配体偶联产物的阴离子识别研究
Re(CO)5 Cl与4-巯基吡啶(4-PySH)的反应导致形成[Re(CO)3(4-HPyS)3 ] Cl(1),显示出三个氢键供体的4- PySH配体以及特征性配体到金属的电荷转移吸收约为。380海里 在这一点上,各种阴离子的,即,CN -,OAC -,F - ,氯- ,溴- ,我-,PF 6 -,NO 3 -,CLO 4 - ,和H 2 PO 4 -进行了研究,以通过氢键功能研究阴离子识别研究。在络合物1的甲醇溶液中添加CN –后,光谱的显着变化,等渗点约为ca。在吸收光谱中观察到314 nm,结合常数(K b)计算为24770 M –1。此外,OAC -阴离子也显示了类似的趋势,但有轻度的光谱变化,ķ b计算为2170中号-1。不像那些CN的-和OAC - ,增加的F - ,氯- ,溴-和我–阴离子引起不太明显的光谱变化,等渗点为ca。350 nm和K b计算为2863–750 M –1。然而,几乎没有光谱变化可以为其他阴离子观察(即,PF 6 -,NO 3 -,H 2 PO 4 - ,和C10 4 - )。有趣的是,[Re(CO)3 Cl(Py 2 S 2)] 2(2 ; Py 2 S 2 = 4,4'-二吡啶基二硫化物)和[Re(CO)3 Cl(Py 2 S)0.35(Py 2 S 2)0.65 ] 2(3 ; Py 2 S = 4,4'-二吡啶硫醚)可以通过X射线衍射分离和结构表征,其中那些晶体是从乙醚扩散到甲醇溶液中生长的分别具有[Bu 4 N] CN和[Bu 4 N] NO 3的配合物1的结构。注意,在这项研究中,可以在室温下实现对配合物2和3的同配体和杂配体环的这种不寻常的配体偶联反应。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号