Chemical Geology ( IF 3.6 ) Pub Date : 2021-03-13 , DOI: 10.1016/j.chemgeo.2021.120176 Wenting Wang , Mengchang He , Wei Ouyang , Chunye Lin , Xitao Liu
|
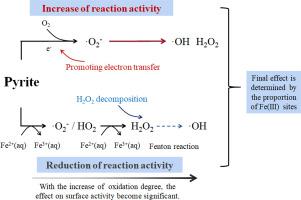
Numerous studies have reported that the atmospheric surface oxidation of pyrite profoundly affects its surface characterization and species. However, controversy remains regarding the influence of atmospheric surface oxidation on the reaction activity of pyrite. Kinetic experiments, the Monte Carlo (MC) simulation, and a kinetic model were used to comprehensively investigate the effect of atmospheric surface oxidation on the production of hydroxyl radicals (·OH) and hydrogen peroxide (H2O2) by pyrite and its reaction activity. The production of ·OH and H2O2 by dissolved Fe(II) (Fe(II)aq) in the suspensions of surface-oxidized pyrite (SOP) was impeded. The primary reason is that Fe (hydr)oxide on the SOP surface can decompose H2O2 to H2O, and the production of ·OH via the Fenton reaction was inhibited subsequently. However, the production of ·OH and H2O2 by Fe(II) sites on the SOP surface (Fe(II)pyrite) was promoted because Fe(III) sites in Fe (hydr)oxide (Fe(III)oxide) enhanced the electron transfer from Fe(II)pyrite to O2. The proportion of Fe(III) sites determined the degree of influence on the production of ·OH and H2O2 by Fe(II)aq and Fe(II)pyrite. The Sb(III) oxidation reaction also indicated that atmospheric surface oxidation altered the oxidation activity of pyrite. In conclusion, atmospheric surface oxidation has a significant effect on the environmental behavior of pyrite and the fate of redox-active substances.
中文翻译:

大气表面氧化对黄铁矿-水界面H 2 O 2和∙OH形成的影响:机理和动力学模型
大量研究报告称,黄铁矿的大气表面氧化深刻影响其表面特征和物种。然而,关于大气表面氧化对黄铁矿反应活性的影响仍存在争议。通过动力学实验,蒙特卡洛模拟(MC)和动力学模型,全面研究了大气表面氧化对黄铁矿产生羟基自由基(·OH)和过氧化氢(H 2 O 2)及其反应的影响活动。溶解的Fe(II)(Fe(II)水溶液生成·OH和H 2 O 2)在表面氧化的黄铁矿(SOP)的悬浮液中被阻止。主要原因是SOP表面的Fe(氢)氧化物可以将H 2 O 2分解为H 2 O,随后抑制了通过Fenton反应产生的·OH。然而,生产的·OH和H 2 ö 2由SOP表面上的Fe(II)位点铁(Fe(II)黄铁矿)中的溶液促进,因为铁(III)中的Fe位点(氢)氧化物铁(Fe(III)氧化物)增强了电子从Fe(II)黄铁矿到O 2的转移。Fe(III)位点的比例决定了Fe(II)水溶液和Fe(II)黄铁矿对·OH和H 2 O 2产生的影响程度。Sb(III)氧化反应还表明,大气表面氧化改变了黄铁矿的氧化活性。总之,大气表面氧化对黄铁矿的环境行为和氧化还原活性物质的命运有重大影响。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号