当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superoxide radical scavenging by sodium 4,5-dihydroxybenzene-1,3-disulfonate dissolved in water: Experimental and quantum chemical studies
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-03-13 , DOI: 10.1016/j.molliq.2021.115810 Sergei O. Liubimovskii , Leila Yu. Ustynyuk , Alexander N. Tikhonov
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2021-03-13 , DOI: 10.1016/j.molliq.2021.115810 Sergei O. Liubimovskii , Leila Yu. Ustynyuk , Alexander N. Tikhonov
|
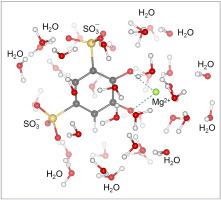
Tiron (sodium 4,5-dihydroxybenzene-1,3-disulfonate) is often used as a probe for detection of superoxide radicals. In this work, we have studied the interaction of Tiron with superoxide radicals dissolved in water solutions. The density functional theory (DFT) method was used for scrutinizing electron and energy characteristics of Tiron radicals in the systems modeling freshwater and salt water. In water solution at neutral pH, deprotonated form of superoxide radical, O2 •- , dominates over the protonated form HO2 • . Fully reduced (TH2 ) and fully oxidized (T) species of Tiron react with superoxide radicals, leading to the formation of the Tiron semiquinone radicals. DFT calculations show that the reactions of O2 •- with TH2 and T in water solution will advance if these reactions are catalyzed by hydronium ions: TH2 + O2 •- + H3 O+ → TH• + H2 O2 + H2 O and T + O2 •- + H3 O+ → TH• + O2 + H2 O. Electron paramagnetic resonance (EPR) and DFT data show that Tiron radicals dissolved in water solution with neutral pH exist predominantly in deprotonated state T•- . The constants of the hyperfine coupling between an unpaired electron and the hydrogen atoms of deprotonated radicals T•- , computed for freshwater and seawater solutions, are in a fairly good agreement with experimental constants derived from the EPR spectra of Tiron radicals.
中文翻译:

溶于水中的 4,5-二羟基苯-1,3-二磺酸钠清除超氧自由基:实验和量子化学研究
Tiron(4,5-二羟基苯-1,3-二磺酸钠)通常用作检测超氧自由基的探针。在这项工作中,我们研究了 Tiron 与溶解在水溶液中的超氧自由基的相互作用。密度泛函理论 (DFT) 方法用于仔细检查淡水和咸水建模系统中 Tiron 自由基的电子和能量特性。在中性 pH 值的水溶液中,超氧自由基的去质子化形式 O2•- 比质子化形式 HO2•占主导地位。完全还原 (TH2) 和完全氧化 (T) 的 Tiron 物质与超氧自由基反应,导致形成 Tiron 半醌自由基。DFT 计算表明,如果这些反应由水合氢离子催化,O2•- 与 TH2 和 T 在水溶液中的反应将提前:TH2 + O2•- + H3O+ → TH• + H2O2 + H2O 和 T + O2•- + H3O+ → TH• + O2 + H2O。电子顺磁共振 (EPR) 和 DFT 数据表明,溶解在中性 pH 值水溶液中的 Tiron 自由基主要以去质子化态 T•- 存在。针对淡水和海水溶液计算的不成对电子和去质子化自由基 T•- 的氢原子之间的超精细耦合常数与从 Tiron 自由基的 EPR 光谱得出的实验常数相当吻合。
更新日期:2021-03-13
中文翻译:

溶于水中的 4,5-二羟基苯-1,3-二磺酸钠清除超氧自由基:实验和量子化学研究
Tiron(4,5-二羟基苯-1,3-二磺酸钠)通常用作检测超氧自由基的探针。在这项工作中,我们研究了 Tiron 与溶解在水溶液中的超氧自由基的相互作用。密度泛函理论 (DFT) 方法用于仔细检查淡水和咸水建模系统中 Tiron 自由基的电子和能量特性。在中性 pH 值的水溶液中,超氧自由基的去质子化形式 O2•- 比质子化形式 HO2•占主导地位。完全还原 (TH2) 和完全氧化 (T) 的 Tiron 物质与超氧自由基反应,导致形成 Tiron 半醌自由基。DFT 计算表明,如果这些反应由水合氢离子催化,O2•- 与 TH2 和 T 在水溶液中的反应将提前:TH2 + O2•- + H3O+ → TH• + H2O2 + H2O 和 T + O2•- + H3O+ → TH• + O2 + H2O。电子顺磁共振 (EPR) 和 DFT 数据表明,溶解在中性 pH 值水溶液中的 Tiron 自由基主要以去质子化态 T•- 存在。针对淡水和海水溶液计算的不成对电子和去质子化自由基 T•- 的氢原子之间的超精细耦合常数与从 Tiron 自由基的 EPR 光谱得出的实验常数相当吻合。

































 京公网安备 11010802027423号
京公网安备 11010802027423号