当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Impact of Antimony(V) on Iron(II)-Catalyzed Ferrihydrite Transformation Pathways: A Novel Mineral Switch for Feroxyhyte Formation
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-03-12 , DOI: 10.1021/acs.est.0c08660 Kerstin Hockmann 1 , Niloofar Karimian 2 , Sara Schlagenhauff 3, 4 , Britta Planer-Friedrich 3 , Edward D. Burton 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2021-03-12 , DOI: 10.1021/acs.est.0c08660 Kerstin Hockmann 1 , Niloofar Karimian 2 , Sara Schlagenhauff 3, 4 , Britta Planer-Friedrich 3 , Edward D. Burton 2
Affiliation
|
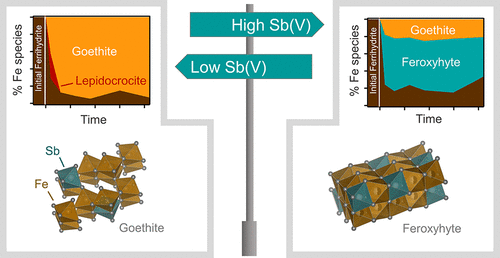
The environmental mobility of antimony (Sb) is controlled by interactions with iron (Fe) oxides, such as ferrihydrite. Under near-neutral pH conditions, Fe(II) catalyzes the transformation of ferrihydrite to more stable phases, thereby potentially altering the partitioning and speciation of associated Sb. Although largely unexplored, Sb itself may also influence ferrihydrite transformation pathways. Here, we investigated the impact of Sb on the Fe(II)-induced transformation of ferrihydrite at pH 7 across a range of Sb(V) loadings (Sb:Fe(III) molar ratios of 0, 0.003, 0.016, and 0.08). At low and medium Sb loadings, Fe(II) induced rapid transformation of ferrihydrite to goethite, with some lepidocrocite forming as an intermediate phase. In contrast, the highest Sb:Fe(III) ratio inhibited lepidocrocite formation, decreased the extent of goethite formation, and instead resulted in substantial formation of feroxyhyte, a rarely reported FeOOH polymorph. At all Sb loadings, the transformation of ferrihydrite was paralleled by a decrease in aqueous and phosphate-extractable Sb concentrations. Extended X-ray absorption fine structure spectroscopy showed that this Sb immobilization was attributable to incorporation of Sb into Fe(III) octahedral sites of the neo-formed minerals. Our results suggest that Fe oxide transformation pathways in Sb-contaminated systems may strongly differ from the well-known pathways under Sb-free conditions.
中文翻译:

锑对铁催化的水铁矿转化途径的影响:铁矿酸盐形成的新型矿物开关。
锑(Sb)的环境迁移率是通过与三氧化二铁(例如三水铁矿)的相互作用来控制的。在接近中性的pH条件下,Fe(II)催化亚铁水合物转变为更稳定的相,从而潜在地改变了相关Sb的分配和形态。尽管很大程度上尚未开发,但Sb本身也可能影响水铁矿的转化途径。在这里,我们研究了在一定的Sb(V)负载量(Sb:Fe(III)摩尔比分别为0、0.003、0.016和0.08)下,Sb对Fe(II)诱导的水铁矿转变的影响(pH 7)。 。在低和中等的锑含量下,Fe(II)引起水铁矿快速转变为针铁矿,并形成了一些锂铁云母作为中间相。相比之下,最高的Sb:Fe(III)比可抑制纤铁矿的形成,减少针铁矿形成的程度,而导致铁氧体的大量形成,这是很少报道的FeOOH多晶型物。在所有Sb负载下,亚铁酸盐的转变与水和磷酸盐可萃取Sb浓度的降低平行。扩展的X射线吸收精细结构光谱表明,这种Sb的固定化归因于Sb掺入新形成的矿物的Fe(III)八面体位点。我们的结果表明,在无Sb污染的系统中,Fe氧化物的转化途径可能与众所周知的途径有很大的不同。水铁矿和磷酸盐可萃取的Sb浓度降低同时伴随着水铁矿的转变。扩展的X射线吸收精细结构光谱表明,这种Sb的固定化归因于Sb掺入新形成的矿物的Fe(III)八面体位点。我们的结果表明,在无Sb污染的系统中,Fe氧化物的转化途径可能与众所周知的途径有很大的不同。水铁矿和磷酸盐可萃取的Sb浓度降低同时伴随着水铁矿的转变。扩展的X射线吸收精细结构光谱表明,这种Sb的固定化归因于Sb掺入新形成的矿物的Fe(III)八面体位点。我们的结果表明,在无Sb污染的系统中,Fe氧化物的转化途径可能与众所周知的途径有很大的不同。
更新日期:2021-04-20
中文翻译:

锑对铁催化的水铁矿转化途径的影响:铁矿酸盐形成的新型矿物开关。
锑(Sb)的环境迁移率是通过与三氧化二铁(例如三水铁矿)的相互作用来控制的。在接近中性的pH条件下,Fe(II)催化亚铁水合物转变为更稳定的相,从而潜在地改变了相关Sb的分配和形态。尽管很大程度上尚未开发,但Sb本身也可能影响水铁矿的转化途径。在这里,我们研究了在一定的Sb(V)负载量(Sb:Fe(III)摩尔比分别为0、0.003、0.016和0.08)下,Sb对Fe(II)诱导的水铁矿转变的影响(pH 7)。 。在低和中等的锑含量下,Fe(II)引起水铁矿快速转变为针铁矿,并形成了一些锂铁云母作为中间相。相比之下,最高的Sb:Fe(III)比可抑制纤铁矿的形成,减少针铁矿形成的程度,而导致铁氧体的大量形成,这是很少报道的FeOOH多晶型物。在所有Sb负载下,亚铁酸盐的转变与水和磷酸盐可萃取Sb浓度的降低平行。扩展的X射线吸收精细结构光谱表明,这种Sb的固定化归因于Sb掺入新形成的矿物的Fe(III)八面体位点。我们的结果表明,在无Sb污染的系统中,Fe氧化物的转化途径可能与众所周知的途径有很大的不同。水铁矿和磷酸盐可萃取的Sb浓度降低同时伴随着水铁矿的转变。扩展的X射线吸收精细结构光谱表明,这种Sb的固定化归因于Sb掺入新形成的矿物的Fe(III)八面体位点。我们的结果表明,在无Sb污染的系统中,Fe氧化物的转化途径可能与众所周知的途径有很大的不同。水铁矿和磷酸盐可萃取的Sb浓度降低同时伴随着水铁矿的转变。扩展的X射线吸收精细结构光谱表明,这种Sb的固定化归因于Sb掺入新形成的矿物的Fe(III)八面体位点。我们的结果表明,在无Sb污染的系统中,Fe氧化物的转化途径可能与众所周知的途径有很大的不同。

































 京公网安备 11010802027423号
京公网安备 11010802027423号