Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2021-03-11 , DOI: 10.1016/j.cej.2021.129306
Chiara Pischetola , Alicia Ruiz-Ruiz , Fernando Cárdenas-Lizana
|
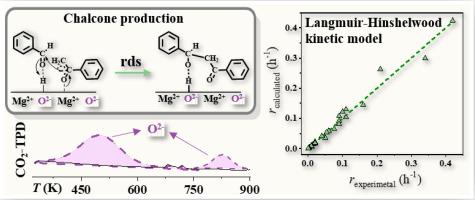
The continuous gas phase condensation of acetophenone (A) with benzaldehyde (B) into valuable (chalcone) benzylideneacetophenone (P = 1 atm, 498 K ≤ T ≤ 573 K; Eapp = 58 ± 4 kJ mol−1) has been performed over an array of commercial oxides (i.e. SiO2, ZnO, ZrO2, CeO2 and MgO) with modified crystal size (18–50 nm, from XRD), specific surface area (8–176 m2 g−1) and total surface basicity (based on carbon dioxide temperature programmed desorption (CO2-TPD)). Reaction operation under chemical controlled regime has been expressly established by parameter estimation and experimental variation of contact time and catalyst/reactant ratio. Full selectivity to target benzylideneacetophenone was achieved over ZnO, ZrO2 and MgO, while benzaldehyde disproportionation to benzyl alcohol and benzoic acid (Cannizzaro reaction) was promoted using SiO2 and CeO2. A direct correlation between activity and specific (per m2) Lewis catalyst basicity has been presented, where MgO delivered the highest chalcone production rate. The reaction orders with respect to acetophenone and benzaldehyde have been estimated, while the experimentally determined benzylideneacetophenone production rates/PA/PB profiles were subjected to a Langmuir-Hinshelwood type kinetic modelling. The best fit was obtained with a model involving non-competitive adsorption of A and B with the surface -C–C- bond formation as rate-determining. Our results demonstrate, for the first time, the sole formation of benzylideneacetophenone over extended reaction time (28 days on-stream) through an alternative continuous gas phase route involving acetophenone + benzaldehyde condensation using MgO to deliver an order of magnitude greater productivity relative to conventional batch liquid systems.
中文翻译:

通过苯甲醛和苯乙酮的气相反应连续生产苄叉基苯乙酮:机理和反应动力学
与苯甲醛(B)转化为有价值的(查耳酮)benzylideneacetophenone(苯乙酮的连续气相缩合(A)P = 1大气压,498ķ≤ Ť ;≤573K的Ê应用 = 58±4千焦耳摩尔-1已经执行过)商业氧化物的阵列(即二氧化硅2,氧化锌,的ZrO 2,的CeO 2和MgO)具有修饰的结晶尺寸(18-50纳米,从XRD),比表面积(8-176米2克-1)和总表面碱度(基于二氧化碳温度编程的解吸量(CO 2-TPD))。通过参数估计以及接触时间和催化剂/反应物比例的实验变化,已经明确建立了化学控制下的反应操作。相对于ZnO,ZrO 2和MgO,对目标亚苄基苯乙酮具有完全选择性,而使用SiO 2和CeO 2则可促进苯甲醛歧化为苯甲醇和苯甲酸(Cannizzaro反应)。已经提出了活性和特定的(每m 2)路易斯催化剂碱度之间的直接关系,其中MgO的查耳酮产率最高。估计了有关苯乙酮和苯甲醛的反应顺序,而实验确定的亚苄基苯乙酮产率/P甲/ P乙型材进行朗缪尔-欣谢尔伍德键入动力学模型。用涉及模型获得的最佳拟合非A的-competitive吸附和B与表面-C-C-键的形成作为速率决定。我们的结果首次证明,通过延长的反应时间(运行28天)唯一形成苄叉基苯乙酮,这是通过使用MgO进行苯乙酮+苯甲醛缩合的另一种连续气相路线实现的,相对于常规方法,生产率提高了一个数量级。分批液体系统。































 京公网安备 11010802027423号
京公网安备 11010802027423号