当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrocatalytic Efficiency of the Oxidation of Ethylene Glycol, Glycerol, and Glucose under Oscillatory Regime
Energy & Fuels ( IF 5.3 ) Pub Date : 2021-03-11 , DOI: 10.1021/acs.energyfuels.1c00203
Gabriel B. Melle , Thiago Altair , Rafael L. Romano , Hamilton Varela
Energy & Fuels ( IF 5.3 ) Pub Date : 2021-03-11 , DOI: 10.1021/acs.energyfuels.1c00203
Gabriel B. Melle , Thiago Altair , Rafael L. Romano , Hamilton Varela
|
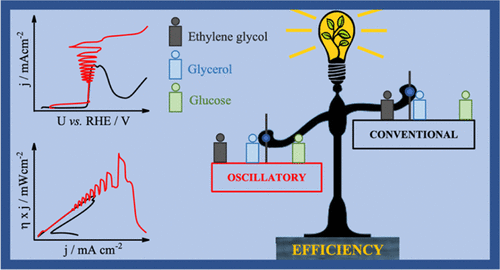
There is an increasing interest in the use of small organic molecules in the interconversion between chemical and electrical energies. Among the strategies to improve the processes of yielding electrical energy in fuel cells and the production of cleaner hydrogen in electrochemical reform, there is the use of kinetic instabilities to improve the conversion and selectivity. Herein, we report on the electrocatalytic efficiency of the oxidation of ethylene glycol, glycerol, and glucose, under regular and oscillatory regimes, on polycrystalline platinum, in sulfuric acid aqueous solution, and at 25 °C. Despite the high overpotentials for the electro-oxidation of these molecules, the electrochemical activity along quasi-stationary potentio-/galvanostatic experiments evidenced that, in all cases, relatively lower potential values, and thus higher activity, are reached during oscillations. Noticeably, higher power densities for the electro-oxidation of ethylene glycol and glycerol under the oscillatory regime were found in a hypothetical direct liquid fuel cell. The use of identical experimental conditions of that of our previous study [J. Phys. Chem. C,2016,120, 22365] allowed to discuss some universal trends for seven small organic molecules. We compile the results in terms of the peak current, the maximum poisoning rate found along the oscillations, and the oscillation frequency. The three parameters were found to decrease in the order: formaldehyde > formic acid > methanol > ethanol > ethylene glycol > glycerol > glucose. In addition, we discussed the increase of the voltammetric current due to the self-organized poisoning rate and reinforced the trend that high electrocatalytic activity implies high susceptibility to surface poisoning for this set of species. Finally, the analysis done for all species (formic acid, formaldehyde, methanol, ethylene glycol, ethanol, glycerol, and glucose) adds to the available thermodynamic data and is a benchmark against which the activities under the oscillatory regime at 25 °C may be compared or assessed. This point of reference permits to explore further experimental conditions that are relevant for energy-related devices, including the conversion of chemical into electrical energy and the electrochemical reform to produce clean hydrogen in electrolyzers.
中文翻译:

振荡条件下乙二醇,甘油和葡萄糖氧化的电催化效率
在化学能和电能之间的相互转换中,越来越多地使用有机小分子。在改善燃料电池中产生电能的过程和电化学改造中产生更清洁的氢的过程的策略中,存在动力学不稳定的使用,以提高转化率和选择性。在此,我们报道了在规则和振荡状态下,在多晶铂上,在硫酸水溶液中以及在25°C下,乙二醇,甘油和葡萄糖氧化的电催化效率。尽管这些分子的电氧化具有很高的过电势,但沿准静态恒电位/恒电流实验的电化学活性表明,在所有情况下,相对较低的电势值,从而在振荡过程中达到更高的活动性。明显地,在假设的直接液体燃料电池中发现了在振荡状态下乙二醇和甘油的电氧化的更高功率密度。使用与我们先前研究相同的实验条件[J.物理 化学 C ,2016,120,[22365]允许讨论七个小有机分子的普遍趋势。我们根据峰值电流,沿着振荡找到的最大中毒率以及振荡频率来汇编结果。发现三个参数按以下顺序降低:甲醛>甲酸>甲醇>乙醇>乙二醇>甘油>葡萄糖。此外,我们讨论了由于自组织中毒率导致的伏安电流的增加,并加强了这样的趋势,即高电催化活性暗示着这组物种对表面中毒的高度敏感性。最后,对所有物种(甲酸,甲醛,甲醇,乙二醇,乙醇,甘油,和葡萄糖)添加到可用的热力学数据中,是可以比较或评估25°C振荡状态下活性的基准。该参考点允许探索与能量相关设备相关的其他实验条件,包括将化学物质转化为电能,以及进行电化学改造以在电解槽中产生干净的氢气。
更新日期:2021-04-01
中文翻译:

振荡条件下乙二醇,甘油和葡萄糖氧化的电催化效率
在化学能和电能之间的相互转换中,越来越多地使用有机小分子。在改善燃料电池中产生电能的过程和电化学改造中产生更清洁的氢的过程的策略中,存在动力学不稳定的使用,以提高转化率和选择性。在此,我们报道了在规则和振荡状态下,在多晶铂上,在硫酸水溶液中以及在25°C下,乙二醇,甘油和葡萄糖氧化的电催化效率。尽管这些分子的电氧化具有很高的过电势,但沿准静态恒电位/恒电流实验的电化学活性表明,在所有情况下,相对较低的电势值,从而在振荡过程中达到更高的活动性。明显地,在假设的直接液体燃料电池中发现了在振荡状态下乙二醇和甘油的电氧化的更高功率密度。使用与我们先前研究相同的实验条件[J.物理 化学 C ,2016,120,[22365]允许讨论七个小有机分子的普遍趋势。我们根据峰值电流,沿着振荡找到的最大中毒率以及振荡频率来汇编结果。发现三个参数按以下顺序降低:甲醛>甲酸>甲醇>乙醇>乙二醇>甘油>葡萄糖。此外,我们讨论了由于自组织中毒率导致的伏安电流的增加,并加强了这样的趋势,即高电催化活性暗示着这组物种对表面中毒的高度敏感性。最后,对所有物种(甲酸,甲醛,甲醇,乙二醇,乙醇,甘油,和葡萄糖)添加到可用的热力学数据中,是可以比较或评估25°C振荡状态下活性的基准。该参考点允许探索与能量相关设备相关的其他实验条件,包括将化学物质转化为电能,以及进行电化学改造以在电解槽中产生干净的氢气。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号