当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Azulene-Based π-Functional Materials: Design, Synthesis, and Applications
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-03-11 , DOI: 10.1021/acs.accounts.0c00893 Hanshen Xin 1 , Bin Hou 1 , Xike Gao 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2021-03-11 , DOI: 10.1021/acs.accounts.0c00893 Hanshen Xin 1 , Bin Hou 1 , Xike Gao 1
Affiliation
|
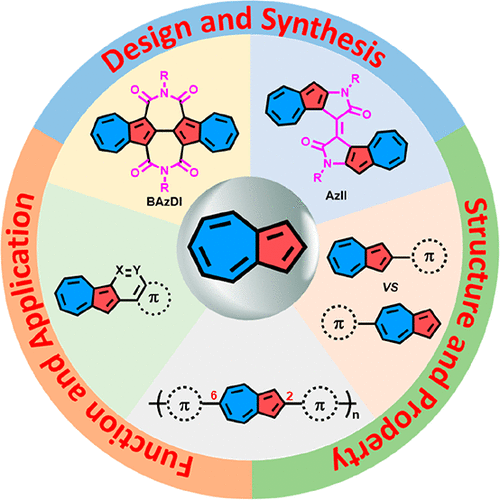
Azulene, an isomer of naphthalene, is a molecule of historical interest for its unusual photophysical properties, including a beautiful blue color derived from the narrow HOMO–LUMO energy gap and anti-Kasha fluorescence from S2 to S0. More recently, it has attracted increasing attention for its novel electronic structure, including an electron-rich five-membered ring and an electron-deficient seven-membered ring with a dipole moment of 1.08 D resulting from resonance delocalization, its different reactivities at odd and even positions, and its stimuli-responsive behavior. As a key building block, azulene has been used in various fields because of its unique physicochemical properties. Recent studies have demonstrated the great potential of azulene for constructing advanced organic materials. However, exploring azulene-based materials has long been hindered by challenges in molecular design and synthesis. Most of the reported azulene-based materials have the azulene unit incorporated through the five-membered ring or seven-membered ring. Creating azulene-based novel building blocks for optoelectronics and using 2,6-connected azulene units to construct conjugated polymers that can adequately utilize the “donor–acceptor” structure of azulene remained underexplored before our contributions. Besides, for most azulene-fused polycyclic aromatic hydrocarbons (PAHs) and heteroaromatics, the azulene substructures were created during later synthesis stages, and the use of azulene derivatives as starting materials to design and synthesize PAHs and heteroaromatics intelligently is still limited.
中文翻译:

基于Azulene的π功能材料:设计,合成和应用
Azulene是萘的一种异构体,由于其不寻常的光物理特性而受到了历史关注,包括从狭窄的HOMO-LUMO能隙和从S 2到S 0的抗-Kasha荧光产生的美丽的蓝色。。最近,它因其新颖的电子结构而引起了越来越多的关注,其中包括一个富电子的五元环和一个缺电子的七元环,由于共振离域,偶极矩为1.08 D,在奇数和奇数时的反应性不同。甚至位置,及其刺激反应行为。作为一种重要的构建基块,由于其独特的理化特性,a嗪已被用于各个领域。最近的研究表明,a石在构建先进的有机材料方面具有巨大的潜力。然而,长期以来,探索基于蓝晶的材料一直受到分子设计和合成挑战的阻碍。所报道的大多数基于z烯的材料具有通过五元环或七元环结合的z烯单元。创造用于光电学的基于氮杂环丁烷的新型构建基块,并使用2,6-连接的氮杂环丁烷单元来构建可以充分利用氮杂环丁烷的“供体-受体”结构的共轭聚合物,在我们做出贡献之前尚未得到充分的研究。此外,对于大多数氮杂稠合的多环芳烃(PAH)和杂芳族化合物而言,在随后的合成阶段会形成氮杂亚结构,并且仍然将氮杂苯衍生物用作起始材料来智能地设计和合成PAH和杂芳族化合物。
更新日期:2021-04-06
中文翻译:

基于Azulene的π功能材料:设计,合成和应用
Azulene是萘的一种异构体,由于其不寻常的光物理特性而受到了历史关注,包括从狭窄的HOMO-LUMO能隙和从S 2到S 0的抗-Kasha荧光产生的美丽的蓝色。。最近,它因其新颖的电子结构而引起了越来越多的关注,其中包括一个富电子的五元环和一个缺电子的七元环,由于共振离域,偶极矩为1.08 D,在奇数和奇数时的反应性不同。甚至位置,及其刺激反应行为。作为一种重要的构建基块,由于其独特的理化特性,a嗪已被用于各个领域。最近的研究表明,a石在构建先进的有机材料方面具有巨大的潜力。然而,长期以来,探索基于蓝晶的材料一直受到分子设计和合成挑战的阻碍。所报道的大多数基于z烯的材料具有通过五元环或七元环结合的z烯单元。创造用于光电学的基于氮杂环丁烷的新型构建基块,并使用2,6-连接的氮杂环丁烷单元来构建可以充分利用氮杂环丁烷的“供体-受体”结构的共轭聚合物,在我们做出贡献之前尚未得到充分的研究。此外,对于大多数氮杂稠合的多环芳烃(PAH)和杂芳族化合物而言,在随后的合成阶段会形成氮杂亚结构,并且仍然将氮杂苯衍生物用作起始材料来智能地设计和合成PAH和杂芳族化合物。






























 京公网安备 11010802027423号
京公网安备 11010802027423号