Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2021-03-10 , DOI: 10.1016/j.jechem.2021.02.026 Bo Zhou , Chung-Li Dong , Yu-Cheng Huang , Nana Zhang , Yandong Wu , Yuxuan Lu , Xu Yue , Zhaohui Xiao , Yuqin Zou , Shuangyin Wang
|
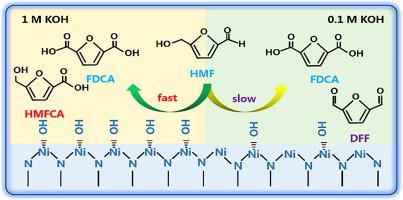
Electro-oxidation of 5-hydroxymethylfurfural (HMFOR) is a promising green approach to realize the conversion of biomass into value-added chemicals. However, considering the complexity of the molecular structure of HMF, an in-depth understanding of the electrocatalytic behavior of HMFOR has rarely been investigated. Herein, the electrocatalytic mechanism of HMFOR on nickel nitride (Ni3N) is elucidated by operando X-ray absorption spectroscopy (XAS), in situ Raman, quasi in situ X-ray photoelectron spectroscopy (XPS), and operando electrochemical impedance spectroscopy (EIS), respectively. The activity origin is proved to be Ni2+δN(OH)ads generated by the adsorbed hydroxyl group. Moreover, HMFOR on Ni3N relates to a two-step reaction: Initially, the applied potential drives Ni atoms to lose electrons and adsorb OH− after 1.35 VRHE, giving rise to Ni2+δN(OH)ads with the electrophilic oxygen; then Ni2+δN(OH)ads seizes protons and electrons from HMF and leaves as H2O spontaneously. Furthermore, the high electrolyte alkalinity favors the HMFOR process due to the increased active species (Ni2+δN(OH)ads) and the enhanced adsorption of HMF on the Ni3N surface. This work could provide an in-depth understanding of the electrocatalytic mechanism of HMFOR on Ni3N and demonstrate the alkalinity effect of the electrolyte on the electrocatalytic performance of HMFOR.
中文翻译:

电催化生物质氧化对氮化镍的活性起源和碱度效应
5-羟甲基糠醛(HMFOR)的电氧化是一种有前途的绿色方法,可实现将生物质转化为增值化学品。但是,考虑到HMF分子结构的复杂性,很少研究对HMFOR的电催化行为的深入了解。这里,HMFOR对氮化镍的电机构(倪3 N)是由operando X射线吸收光谱(XAS)阐明,原位拉曼,准原位X射线光电子能谱(XPS),以及operando电化学阻抗谱( EIS)。活性来源被证明是Ni 2 + δN(OH)ad由吸附的羟基产生。此外,在Ni HMFOR 3 Ñ涉及一种两步反应:首先,施加的电势驱动Ni原子失去电子并吸附OH -后1.35V,RHE,产生的Ni 2+ δ N(OH)广告与亲电氧; 然后镍2+ δ N(OH)广告从HMF掌握到质子和电子和叶以H 2 ö自发。此外,高电解质碱度有利于HMFOR过程由于增加的活性种(镍2+ δ N(OH)广告)和HMF的对Ni增强吸附3N面。这项工作可以提供对HMFOR对Ni 3 N的电催化机理的深入了解,并证明电解质的碱度效应对HMFOR的电催化性能。































 京公网安备 11010802027423号
京公网安备 11010802027423号