当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rapid iodine adsorption from vapor phase and solution by a nitrogen-rich covalent piperazine–triazine-based polymer
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-2-24 , DOI: 10.1039/d1nj00122a Yalin Huang 1, 2, 3, 4 , Wei Li 4, 5, 6, 7 , Yuwei Xu 1, 2, 3, 4 , Mu Ding 1, 2, 3, 4 , Jie Ding 1, 2, 3, 4 , Yun Zhang 1, 2, 3, 4 , Yuanhua Wang 1, 2, 3, 4 , Shanyong Chen 1, 2, 3, 4 , Yongdong Jin 1, 2, 3, 4 , Chuanqin Xia 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2021-2-24 , DOI: 10.1039/d1nj00122a Yalin Huang 1, 2, 3, 4 , Wei Li 4, 5, 6, 7 , Yuwei Xu 1, 2, 3, 4 , Mu Ding 1, 2, 3, 4 , Jie Ding 1, 2, 3, 4 , Yun Zhang 1, 2, 3, 4 , Yuanhua Wang 1, 2, 3, 4 , Shanyong Chen 1, 2, 3, 4 , Yongdong Jin 1, 2, 3, 4 , Chuanqin Xia 1, 2, 3, 4
Affiliation
|
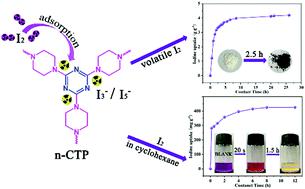
The efficient capture of radioiodine from the waste of nuclear industries is a growing priority for the safe development of nuclear energy. However, because the iodine adsorption rate of previously reported adsorbents is very slow, they are inadequate for use in an actual emergency such as radioiodine leakage in a nuclear accident. Herein, a nitrogen-rich covalent triazine–piperazine-based porous polymer (n-CTP) was successfully synthesized via a mild one-step solvothermal route. The large pore size and abundant N-heterocyclic groups (with a nitrogen content as high as 39.69%) of n-CTP are beneficial for the diffusion and adsorption of I2, and they have the ability to decrease the adsorption time. The ability of n-CTP to adsorb iodine vapor was examined, and the results show that n-CTP possesses a faster adsorption rate (1.58 g g−1 h−1) than most iodine-capturing covalent organic polymers (COPs), excellent iodine uptake capacity (4.19 g g−1), satisfactory selectivity, and can be reused for at least three cycles without a significant loss of iodine uptake. FT-IR and Raman analyses revealed that the mechanism of iodine capture is attributed to the charge transfer interactions between n-CTP and the guest iodine. In addition, n-CTP also exhibits high iodine capture capacity (2.88 g g−1) and fast adsorption rate in iodine solutions. Thus, n-CTP has the potential to be applied to the rapid removal and enrichment of radioiodine in spent fuel reprocessing and has the ability to prevent environmental contamination in the case of nuclear accidents.
中文翻译:

富氮共价哌嗪-三嗪基聚合物从气相和溶液中快速吸附碘
从核工业废物中有效捕集放射性碘是安全发展核能的一个日益重要的优先事项。然而,由于先前报道的吸附剂的碘吸附速率非常慢,因此它们不足以用于实际紧急情况,例如核事故中的放射性碘泄漏。本文中,通过温和的一步溶剂热法成功合成了富氮共价三嗪-哌嗪基多孔聚合物(n-CTP)。n-CTP的大孔径和丰富的N-杂环基(氮含量高达39.69%)有利于I 2的扩散和吸附,并且它们具有减少吸附时间的能力。研究了n-CTP吸附碘蒸气的能力,结果表明,n-CTP的吸附速率(1.58 gg -1 h -1)比大多数捕获碘的共价有机聚合物(COPs)快,碘的吸收能力也很好。容量(4.19 gg -1),令人满意的选择性,并且可以重复使用至少三个周期,而不会显着降低碘的吸收。FT-IR和拉曼分析表明,碘捕获的机制归因于n-CTP与客体碘之间的电荷转移相互作用。此外,n-CTP还具有很高的碘捕获能力(2.88 gg -1)和在碘溶液中的快速吸附速率。因此,n-CTP有潜力应用于乏燃料后处理中放射性碘的快速去除和富集,并具有防止发生核事故的环境污染的能力。
更新日期:2021-03-09
中文翻译:

富氮共价哌嗪-三嗪基聚合物从气相和溶液中快速吸附碘
从核工业废物中有效捕集放射性碘是安全发展核能的一个日益重要的优先事项。然而,由于先前报道的吸附剂的碘吸附速率非常慢,因此它们不足以用于实际紧急情况,例如核事故中的放射性碘泄漏。本文中,通过温和的一步溶剂热法成功合成了富氮共价三嗪-哌嗪基多孔聚合物(n-CTP)。n-CTP的大孔径和丰富的N-杂环基(氮含量高达39.69%)有利于I 2的扩散和吸附,并且它们具有减少吸附时间的能力。研究了n-CTP吸附碘蒸气的能力,结果表明,n-CTP的吸附速率(1.58 gg -1 h -1)比大多数捕获碘的共价有机聚合物(COPs)快,碘的吸收能力也很好。容量(4.19 gg -1),令人满意的选择性,并且可以重复使用至少三个周期,而不会显着降低碘的吸收。FT-IR和拉曼分析表明,碘捕获的机制归因于n-CTP与客体碘之间的电荷转移相互作用。此外,n-CTP还具有很高的碘捕获能力(2.88 gg -1)和在碘溶液中的快速吸附速率。因此,n-CTP有潜力应用于乏燃料后处理中放射性碘的快速去除和富集,并具有防止发生核事故的环境污染的能力。































 京公网安备 11010802027423号
京公网安备 11010802027423号