当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Small-Molecule Targeted Aβ42 Aggregate Degradation: Negatively Charged Small Molecules Are More Promising than the Neutral Ones
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-03-09 , DOI: 10.1021/acschemneuro.1c00047 Jinfei Mei 1 , Huijuan Yang 1 , Bo Sun 1 , Chengqiang Liu 1 , Hongqi Ai 1
ACS Chemical Neuroscience ( IF 4.1 ) Pub Date : 2021-03-09 , DOI: 10.1021/acschemneuro.1c00047 Jinfei Mei 1 , Huijuan Yang 1 , Bo Sun 1 , Chengqiang Liu 1 , Hongqi Ai 1
Affiliation
|
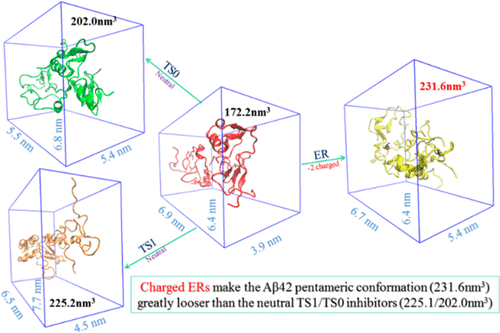
Heavy evidence has confirmed that Aβ42 oligomers are the most neurotoxic aggregates and play a critical role in the occurrence and development of Alzheimer’s disease by causing functional neuron death, cognitive damage, and dementia. Disordered Aβ42 oligomers are challenging therapeutic targets, and no drug is currently in clinical use that modifies the properties of their monomeric states. Here, a negatively charged molecule (ER), rather than the neutral TS1 one, is identified by a molecular dynamics simulation method to be more capable of binding and sequestering the intrinsically disordered amyloid-β peptide Aβ42 in its soluble pentameric state as well as its monomeric components. Results reveal that the ERs interact with Aβ and inhibit the primary nucleation pathways in its aggregation process in entropic expansion mechanism for both Aβ42 and Aβ40 oligomers but with opposite characteristics of hydrophobic surface area (HSA). The interaction between Aβ42 oligomer and either charged ER or neutral TS1/TS0 characterizes decreased HSA, and the decrease in ER-involved case is highly visible, consistent with the observations from in silico and in vitro studies. By contrast, the presence of these inhibitors causes the HSA of Aβ40 oligomer to change undetectably and there is even a bit of increase in the histidine isomerized Aβ40 oligomer. The HSA distinction between Aβ42 and Aβ40 oligomer is possibly derived from the different effects of M35-inhibitor interaction, which is analogous to the effect of M35 oxidation. In comparison with the neutral TS1/TS0 inhibitors, ER is more prone to bind the residues located in the central (β1) and C-terminal (β2) regions of Aβ42 peptide, two key nucleation regions for Aβ intramolecular folding, intermolecular aggregation, and assembly. Notably, ER can strongly bind the charged residues, such as K16, K28, D23, to greatly disturb the potential stabilizer (e.g., salt-bridge, etc.) in metastable Aβ42 oligomers and protofibrils. These results illustrate the strategy of overcoming Alzheimer’s disease from inhibiting its early stage Aβ aggregation with two kinds of small molecules to alter their behavior for therapeutic purposes and strongly recommend paying more attention to the engineering and development of negatively charged inhibitors, the long-term underappreciated ones, targeting the early stage Aβ aggregates.
中文翻译:

小分子靶向Aβ42聚集体降解:带负电荷的小分子比中性分子更有前途
大量证据证实,Aβ42低聚物是最具神经毒性的聚集体,并通过引起功能性神经元死亡,认知损伤和痴呆在阿尔茨海默氏病的发生和发展中发挥关键作用。紊乱的Aβ42低聚物是具有挑战性的治疗目标,目前尚无可改变其单体状态特性的药物在临床上使用。在此,通过分子动力学模拟方法鉴定出带负电荷的分子(ER),而不是中性TS1,能够结合和螯合内在无序的淀粉样β肽Aβ42及其可溶五聚体状态及其螯合状态。单体成分。结果表明,ERs与Aβ相互作用并在其聚集过程中以Aβ42和Aβ40低聚物的熵膨胀机制抑制主要的成核途径,但具有相反的疏水表面积(HSA)特征。Aβ42低聚物与带电的ER或中性TS1 / TS0之间的相互作用表征了HSA的降低,并且与ER和计算机有关的观察结果均与观察到的ER降低有关。相比之下,这些抑制剂的存在导致Aβ40低聚物的HSA无法检测到变化,并且组氨酸异构化的Aβ40低聚物甚至有所增加。Aβ42和Aβ40低聚物之间的HSA区别可能源自M35-抑制剂相互作用的不同作用,这类似于M35氧化的作用。与中性TS1 / TS0抑制剂相比,ER更倾向于结合位于Aβ42肽的中央(β1)和C末端(β2),两个Aβ分子内折叠,分子间聚集和两个关键成核区域的残基。集会。值得注意的是,ER可以牢固地结合带电荷的残基,例如K16,K28,D23,以极大地干扰亚稳的Aβ42寡聚体和原纤维中的潜在稳定剂(例如盐桥等)。这些结果说明了通过两种小分子抑制阿尔茨海默氏病早期Aβ聚集从而改变其行为以达到治疗目的的策略,并强烈建议更加重视带负电荷的抑制剂的设计和开发,长期以来人们对此认识不足。靶向早期Aβ聚集体。
更新日期:2021-04-08
中文翻译:

小分子靶向Aβ42聚集体降解:带负电荷的小分子比中性分子更有前途
大量证据证实,Aβ42低聚物是最具神经毒性的聚集体,并通过引起功能性神经元死亡,认知损伤和痴呆在阿尔茨海默氏病的发生和发展中发挥关键作用。紊乱的Aβ42低聚物是具有挑战性的治疗目标,目前尚无可改变其单体状态特性的药物在临床上使用。在此,通过分子动力学模拟方法鉴定出带负电荷的分子(ER),而不是中性TS1,能够结合和螯合内在无序的淀粉样β肽Aβ42及其可溶五聚体状态及其螯合状态。单体成分。结果表明,ERs与Aβ相互作用并在其聚集过程中以Aβ42和Aβ40低聚物的熵膨胀机制抑制主要的成核途径,但具有相反的疏水表面积(HSA)特征。Aβ42低聚物与带电的ER或中性TS1 / TS0之间的相互作用表征了HSA的降低,并且与ER和计算机有关的观察结果均与观察到的ER降低有关。相比之下,这些抑制剂的存在导致Aβ40低聚物的HSA无法检测到变化,并且组氨酸异构化的Aβ40低聚物甚至有所增加。Aβ42和Aβ40低聚物之间的HSA区别可能源自M35-抑制剂相互作用的不同作用,这类似于M35氧化的作用。与中性TS1 / TS0抑制剂相比,ER更倾向于结合位于Aβ42肽的中央(β1)和C末端(β2),两个Aβ分子内折叠,分子间聚集和两个关键成核区域的残基。集会。值得注意的是,ER可以牢固地结合带电荷的残基,例如K16,K28,D23,以极大地干扰亚稳的Aβ42寡聚体和原纤维中的潜在稳定剂(例如盐桥等)。这些结果说明了通过两种小分子抑制阿尔茨海默氏病早期Aβ聚集从而改变其行为以达到治疗目的的策略,并强烈建议更加重视带负电荷的抑制剂的设计和开发,长期以来人们对此认识不足。靶向早期Aβ聚集体。































 京公网安备 11010802027423号
京公网安备 11010802027423号