当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeted Regulation of Blood–Brain Barrier for Enhanced Therapeutic Efficiency of Hypoxia-Modifier Nanoparticles and Immune Checkpoint Blockade Antibodies for Glioblastoma
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-08 , DOI: 10.1021/acsami.1c00347 Lingtong Meng 1 , Cuirong Wang 1 , Yaping Lu 1 , Gang Sheng 1 , Lin Yang 1 , Zhouyue Wu 1 , Hang Xu 1 , Chao Han 1 , Yingmei Lu 2 , Feng Han 1
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-08 , DOI: 10.1021/acsami.1c00347 Lingtong Meng 1 , Cuirong Wang 1 , Yaping Lu 1 , Gang Sheng 1 , Lin Yang 1 , Zhouyue Wu 1 , Hang Xu 1 , Chao Han 1 , Yingmei Lu 2 , Feng Han 1
Affiliation
|
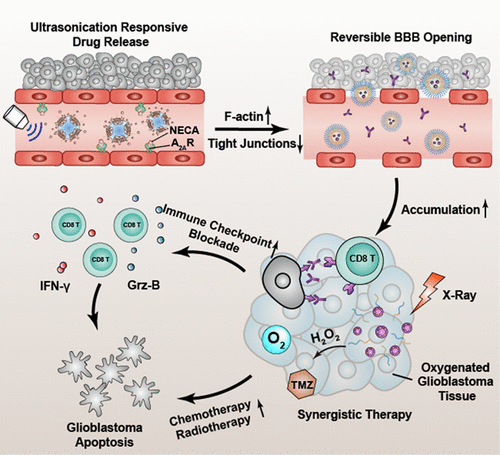
Glioblastoma is the most destructive type of brain cancer. The blood–brain barrier (BBB) is a tremendous obstacle that hinders therapeutic agents, such as chemical drugs and antibodies, from reaching glioblastoma tissues. Meanwhile, the abnormal microenvironment of glioblastoma extremely restricts the expected therapeutic effects of accumulated drugs. Therefore, in the present study, BBB-regulating nanovesicles (BRN) are developed to achieve targeted and controlled BBB regulation, carrying adenosine 2A receptor (A2AR) agonists and perfluorocarbon (PF). The red-blood-cell membrane (RBCM) is included on the outside to avoid the premature release of therapeutic agents. In the presence of ultrasonication (US), A2AR agonists are released and induce effects on both F-actin and tight junctions of endothelial cells. Subsequently, BBB permeability is temporarily increased and enables small molecules and nanoparticles to enter brain parenchymal tissues. The high affinity between manganese dioxide and temozolomide (TMZ) is utilized to form multifunctional nanoparticles to ameliorate the hypoxic microenvironment, which yields improved glioblastoma inhibition combined with radiotherapy. Moreover, with the aid of targeted BBB regulation, programmed death ligand-1 (PD-L1) antibody induces a tumor-specific immune response. Taken together, the findings suggest that synergistic combination may have the potential in amplifying the therapeutic efficacies of clinical drugs and immune checkpoint blockade antibodies to overcome the therapeutic resistance of glioblastoma.
中文翻译:

靶向调节血脑屏障,提高缺氧修饰剂纳米颗粒的治疗效率和胶质母细胞瘤的免疫检查点封锁抗体
胶质母细胞瘤是最具破坏性的脑癌类型。血脑屏障(BBB)是一个巨大的障碍,阻碍了化学药物和抗体等治疗剂到达胶质母细胞瘤组织。同时,胶质母细胞瘤的微环境异常极大地限制了累积药物的预期治疗效果。因此,在本研究中,开发了BBB调节纳米囊泡(BRN),以实现靶向和受控的BBB调节,并带有腺苷2A受体(A 2A R)激动剂和全氟化碳(PF)。红细胞膜(RBCM)包含在外部,以避免治疗剂过早释放。在超声(美国)的情况下,A 2AR激动剂被释放并诱导对F-肌动蛋白和内皮细胞紧密连接的作用。随后,血脑屏障通透性暂时增加,并使小分子和纳米颗粒进入脑实质组织。利用二氧化锰和替莫唑胺(TMZ)之间的高亲和力来形成多功能纳米颗粒,以改善缺氧的微环境,从而结合放射疗法改善胶质母细胞瘤的抑制作用。此外,借助靶向的BBB调节,程序性死亡配体1(PD-L1)抗体可诱导肿瘤特异性免疫反应。综上所述,该发现表明协同组合可能具有扩大临床药物和免疫检查点阻断抗体的治疗功效以克服胶质母细胞瘤的治疗抗性的潜力。
更新日期:2021-03-17
中文翻译:

靶向调节血脑屏障,提高缺氧修饰剂纳米颗粒的治疗效率和胶质母细胞瘤的免疫检查点封锁抗体
胶质母细胞瘤是最具破坏性的脑癌类型。血脑屏障(BBB)是一个巨大的障碍,阻碍了化学药物和抗体等治疗剂到达胶质母细胞瘤组织。同时,胶质母细胞瘤的微环境异常极大地限制了累积药物的预期治疗效果。因此,在本研究中,开发了BBB调节纳米囊泡(BRN),以实现靶向和受控的BBB调节,并带有腺苷2A受体(A 2A R)激动剂和全氟化碳(PF)。红细胞膜(RBCM)包含在外部,以避免治疗剂过早释放。在超声(美国)的情况下,A 2AR激动剂被释放并诱导对F-肌动蛋白和内皮细胞紧密连接的作用。随后,血脑屏障通透性暂时增加,并使小分子和纳米颗粒进入脑实质组织。利用二氧化锰和替莫唑胺(TMZ)之间的高亲和力来形成多功能纳米颗粒,以改善缺氧的微环境,从而结合放射疗法改善胶质母细胞瘤的抑制作用。此外,借助靶向的BBB调节,程序性死亡配体1(PD-L1)抗体可诱导肿瘤特异性免疫反应。综上所述,该发现表明协同组合可能具有扩大临床药物和免疫检查点阻断抗体的治疗功效以克服胶质母细胞瘤的治疗抗性的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号