Results in Chemistry ( IF 2.5 ) Pub Date : 2021-03-09 , DOI: 10.1016/j.rechem.2021.100124 Materu Yuyama , Takashi Misawa , Yosuke Demizu , Takayuki Kanaya , Masaaki Kurihara
|
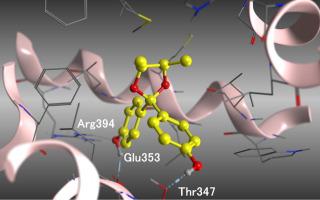
Novel compounds bearing acetal groups in their biphenylmethane skeletons were synthesized in moderate yields from benzophenone derivative. Compound 1 did not exhibit antagonistic activity against the ERα estrogen receptor; however, compounds 2, 3, and 4 exhibited potent ERα antagonistic activities. A small difference in the ERα antagonistic activities of the stereoisomers was observed. It was suggested that the methyl groups on the acetal moieties were responsible for the observed ERα antagonistic activities of the compounds. These results could be attributed to interactions of the methyl groups of the acetal functional groups with the hydrophobic binding residues of the binding site.
中文翻译:

含联苯甲烷骨架乙缩醛的新型雌激素受体拮抗剂的设计与合成
从二苯甲酮衍生物以中等收率合成了在其联苯甲烷骨架上带有缩醛基团的新型化合物。复方1并没有表现出对ER拮抗活性α雌激素受体; 然而,化合物2,3和4显示出强效的ER α拮抗活性。在ER小差α观察到的立体异构体的拮抗活性。有人建议,在缩醛部分的甲基分别负责观察到的ER α化合物的拮抗活性。这些结果可以归因于缩醛官能团的甲基与结合位点的疏水结合残基的相互作用。





























 京公网安备 11010802027423号
京公网安备 11010802027423号