Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2021-03-09 , DOI: 10.1016/j.freeradbiomed.2021.02.031 Yanwen Zhu 1 , Ling Liu 1 , Dehong Tan 1 , Weijie Sun 1 , Qin Ke 2 , Xiqing Yue 1 , Bing Bai 1
|
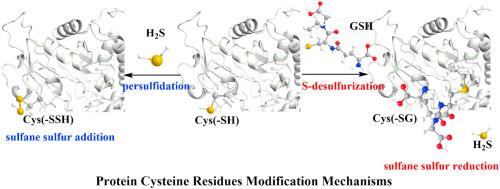
Post-translational transformation of cysteine residues to persulfides, known as protein S-sulfhydration or persulfidation, is a beneficial H2S signaling mechanism. In this paper, we found that GSH is bound to active cysteine sites of protein by S-desulfurization, which is a new covalent modification mechanism of protein, thus regulating catalytic activity. Here, we provide direct evidence that GSH modifies the reactive cysteine residues of four enzymes (alliinase/D-LDH/ADH/G6PD) and generates protein-SG or protein-SSG derivatives by S-desulfurization. S-desulfurization, α-carbon nucleophilic substitution or thiol-disulfide exchange occurs and H2S is released as a by-product. S-desulfurization is the opposite of persulfidation in terms of H2S production/consumption and enzyme inhibition/mitigation. Here, we elucidated the GSH mechanisms and H2S mechanisms in the enzyme-metabolite system and the beneficial roles of persulfidation and S-desulfurization. These theoretical findings are now shedding light on understanding GSH and H2S molecular functions and providing new theoretical basis for them in cell signaling pathways.
中文翻译:

S-脱硫:与GSH过硫化不同的共价修饰机理
半胱氨酸残基的翻译后转化为过硫化物,称为蛋白S-硫酸化或过硫化,是一种有益的H 2 S信号传导机制。在本文中,我们发现GSH通过S-脱硫与蛋白质的活性半胱氨酸位点结合,这是一种新的蛋白质共价修饰机制,从而调节了催化活性。在这里,我们提供直接的证据,表明GSH修饰了四种酶(alliinase / D -LDH / ADH / G6PD)的反应性半胱氨酸残基,并通过S-脱硫产生了蛋白质-SG或蛋白质-SSG衍生物。发生S-脱硫,α-碳亲核取代或硫醇-二硫键交换,H 2 S作为副产物释放。就H 2而言,S脱硫与过硫化相反S的产生/消耗和酶的抑制/缓解。在这里,我们阐明了酶代谢系统中的GSH机理和H 2 S机理以及过硫化和S脱硫的有益作用。这些理论发现为了解GSH和H 2 S分子功能提供了新的理论依据,并为它们在细胞信号传导途径中的应用提供了新的理论基础。






























 京公网安备 11010802027423号
京公网安备 11010802027423号