Chem ( IF 19.1 ) Pub Date : 2021-03-09 , DOI: 10.1016/j.chempr.2021.02.011 David Hess , Veronika Dockalova , Piia Kokkonen , David Bednar , Jiri Damborsky , Andrew deMello , Zbynek Prokop , Stavros Stavrakis
|
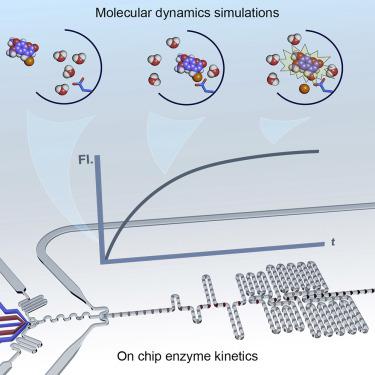
The ability to engineer enzymes for industrial and biomedical applications is primarily limited by a paucity of mechanistic understanding. To gain insight into the mechanisms of enzyme catalysis, one must screen enormous numbers of discrete reaction conditions, which is a laborious task using conventional technologies. To address such limitations, we develop a droplet-based microfluidic platform for high-throughput acquisition of transient kinetic data over a range of substrate concentrations and temperatures. When compared with conventional methods, our platform reduces assay volumes by six orders of magnitude and increases throughput to 9,000 reactions/min. To demonstrate their utility, we measure the transient kinetics of three model enzymes, namely, β-galactosidase, horseradish peroxidase, and microperoxidase. Additionally, we conduct a complex kinetic and thermodynamic study of engineered variants of haloalkane dehalogenases. Datasets are globally analyzed and complemented by molecular dynamics simulations, providing new insights into the molecular basis of substrate specificity and the role of hydration-related entropy.
中文翻译:

片上瞬态动力学结合全局数据分析和分子建模探索酶催化的机理
对于工业和生物医学应用工程化酶的能力主要受到缺乏机械理解的限制。为了深入了解酶催化的机理,必须筛选大量离散的反应条件,这是使用常规技术的一项艰巨的任务。为了解决这些局限性,我们开发了一种基于液滴的微流体平台,用于在一定范围的底物浓度和温度范围内高通量采集瞬态动力学数据。与传统方法相比,我们的平台将测定体积减少了六个数量级,并将通量提高到9,000个反应/分钟。为了证明其实用性,我们测量了三种模型酶(β-半乳糖苷酶,辣根过氧化物酶和微过氧化物酶)的瞬态动力学。此外,我们对卤代烷脱卤酶的工程变体进行了复杂的动力学和热力学研究。对数据集进行了全局分析,并通过分子动力学模拟进行了补充,为底物特异性的分子基础以及与水合有关的熵的作用提供了新的见解。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号