Cell Metabolism ( IF 27.7 ) Pub Date : 2021-03-09 , DOI: 10.1016/j.cmet.2021.02.015 Xingzhe Ma 1 , Liuling Xiao 1 , Lintao Liu 1 , Lingqun Ye 1 , Pan Su 1 , Enguang Bi 1 , Qiang Wang 1 , Maojie Yang 1 , Jianfei Qian 1 , Qing Yi 1
|
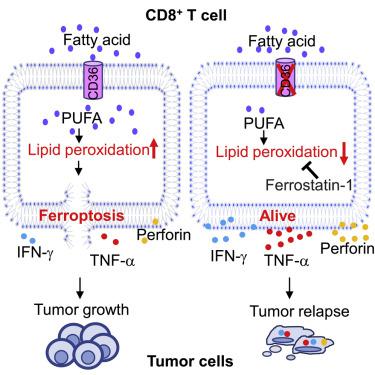
Understanding the mechanisms underlying how T cells become dysfunctional in a tumor microenvironment (TME) will greatly benefit cancer immunotherapy. We found that increased CD36 expression in tumor-infiltrating CD8+ T cells, which was induced by TME cholesterol, was associated with tumor progression and poor survival in human and murine cancers. Genetic ablation of Cd36 in effector CD8+ T cells exhibited increased cytotoxic cytokine production and enhanced tumor eradication. CD36 mediated uptake of fatty acids by tumor-infiltrating CD8+ T cells in TME, induced lipid peroxidation and ferroptosis, and led to reduced cytotoxic cytokine production and impaired antitumor ability. Blocking CD36 or inhibiting ferroptosis in CD8+ T cells effectively restored their antitumor activity and, more importantly, possessed greater antitumor efficacy in combination with anti-PD-1 antibodies. This study reveals a new mechanism of CD36 regulating the function of CD8+ effector T cells and therapeutic potential of targeting CD36 or inhibiting ferroptosis to restore T cell function.
中文翻译:

CD36 介导的铁死亡抑制肿瘤内 CD8+ T 细胞效应功能并削弱其抗肿瘤能力
了解 T 细胞如何在肿瘤微环境 (TME) 中功能失调的机制将极大地有益于癌症免疫治疗。我们发现由 TME 胆固醇诱导的肿瘤浸润性 CD8 + T 细胞中 CD36 表达增加与人类和小鼠癌症的肿瘤进展和较差的存活率有关。效应 CD8 + T 细胞中Cd36的基因消融表现出增加的细胞毒性细胞因子产生和增强的肿瘤根除。CD36 介导 TME 中肿瘤浸润性 CD8 + T 细胞摄取脂肪酸,诱导脂质过氧化和铁死亡,并导致细胞毒性细胞因子产生减少和抗肿瘤能力受损。阻断 CD36 或抑制 CD8 中的铁死亡+ T 细胞有效地恢复了它们的抗肿瘤活性,更重要的是,与抗 PD-1 抗体组合具有更大的抗肿瘤功效。本研究揭示了CD36调节CD8 +效应T细胞功能的新机制以及靶向CD36或抑制铁死亡恢复T细胞功能的治疗潜力。






























 京公网安备 11010802027423号
京公网安备 11010802027423号