Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2021-03-08 , DOI: 10.1016/j.saa.2021.119662
Guowen Zhang , Na Li , Ying Zhang , Junhui Pan , Deming Gong
|
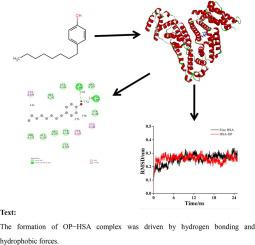
4−Octylphenol (OP) is an environmental estrogen that can enter organisms through the food chain and cause various toxic effects. Here, the interaction between OP and human serum albumin (HSA) was explored through multipectral, molecular docking and dynamics simulation. The results showed that OP and HSA formed a ground state complex through a static quenching mechanism, and the interaction was spontaneously driven by hydrogen bonds and hydrophobic interaction forces. The binding constant at different temperatures was measured to be on the order of 105 L mol−1. Site competition experiments suggested that OP interacted with amino acid residues Lys195, Cy245 and Cys246 located at the Sudlow site I of HSA, resulting in a more stretched protein peptide. The presence of OP increased the surface hydrophobicity of HSA, and reduced the content of α-helix in HSA by 3.4%. FT−IR spectra showed that OP interacted with the C=O and C-H groups of the polypeptide backbone. Molecular docking demonstrated that OP mainly bound to Site I of HSA and hydrogen bonds participated in the interaction. In addition, molecular dynamics simulations further explored the stability and dynamic behavior of the OP−HSA complex through RMSD, RMSF, SASA and Rg.
中文翻译:

4-辛基苯酚与人血清白蛋白的结合机理:光谱研究,分子对接和动力学模拟
4-辛基苯酚(OP)是一种环境雌激素,可通过食物链进入生物并引起各种毒性作用。在这里,OP和人类血清白蛋白(HSA)之间的相互作用通过多角度,分子对接和动力学模拟进行了探讨。结果表明,OP和HSA通过静态猝灭机理形成基态配合物,并且相互作用是由氢键和疏水相互作用力自发驱动的。经测量,在不同温度下的结合常数约为10 5 L mol -1。位点竞争实验表明,OP与位于HSA Sudlow位点I的氨基酸残基Lys195,Cy245和Cys246相互作用,产生了更伸展的蛋白质肽。OP的存在增加了HSA的表面疏水性,并使HSA中的α-螺旋含量降低了3.4%。FT-IR光谱表明,OP与多肽主链的C = O和CH基团相互作用。分子对接表明,主要结合于HSA位点I的OP和氢键参与了相互作用。此外,分子动力学模拟还通过RMSD,RMSF,SASA和Rg探索了OP-HSA复合物的稳定性和动力学行为。

































 京公网安备 11010802027423号
京公网安备 11010802027423号