当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Controlling the Formation of Charge Transfer Complexes in Chemically Doped Semiconducting Polymers
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-03-05 , DOI: 10.1021/acs.chemmater.0c04471
Dane A. Stanfield 1 , Yutong Wu 1 , Sarah H. Tolbert 1, 2 , Benjamin J. Schwartz 1
Chemistry of Materials ( IF 7.2 ) Pub Date : 2021-03-05 , DOI: 10.1021/acs.chemmater.0c04471
Dane A. Stanfield 1 , Yutong Wu 1 , Sarah H. Tolbert 1, 2 , Benjamin J. Schwartz 1
Affiliation
|
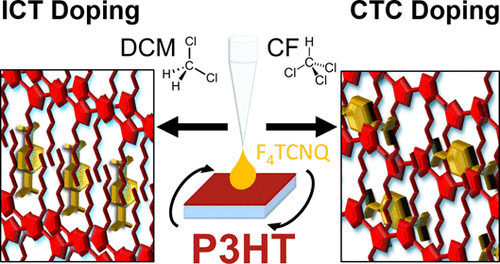
Chemical doping of semiconducting polymers predominantly takes place via integer charge transfer (ICT), where an electron is entirely removed from the host conjugated polymer and transferred to reside on the dopant guest species. In contrast, chemical doping of small conjugated molecules and oligomers often leads to the formation of charge transfer complexes (CTCs), which have significant orbital overlap and shared electron density between the host and guest species. To date, the observation of fractional charge transfer in doped conjugated polymers is relatively rare, occurring only under extreme processing conditions that can be difficult to achieve, which is fortunate given that CTC formation generally yields fewer mobile carriers per dopant. In this work, we use the classic conjugated polymer/dopant pair of P3HT and F4TCNQ to demonstrate how simply adjusting the casting solvent for the dopant in sequential processing can fundamentally alter the nature of doping in this well-studied system, leading to tunable production of CTCs. Using solvent blends of dichloromethane and chloroform, selected for their low and high solubility toward P3HT, respectively, we show that the relative amount of polymer-dopant CTCs can be readily controlled over an order of magnitude. Increasing the amount of chloroform in the dopant solvent blend favors the creation of CTCs, while increasing the dichloromethane content results in doping by the more standard ICT; the results allow us to explain why CTC formation is common in charge-transfer salts but generally less so in doped conjugated polymers. We also explore the role of the doping method and the crystallinity of P3HT films in controlling the relative amounts of ICT and CTC formation. We find that the use of evaporation doping and higher-crystallinity material discourages CTC formation, but that even in the most favorable case of evaporation doping with high polymer crystallinity, fractional charge transfer always occurs to some extent. Finally, we show that brief thermal annealing can convert CTCs to integer charge transfer species, indicating that ICT is the thermodynamically preferred doping mechanism in conjugated polymers, and that fractional charge transfer is the result of kinetic trapping. With this understanding, we offer guidelines for limiting the occurrence of charge transfer complexes during sequential doping of conjugated polymers, thus avoiding the deleterious effects of CTCs on charge transport.
中文翻译:

控制化学掺杂半导体聚合物中电荷转移络合物的形成
半导体聚合物的化学掺杂主要通过整数电荷转移(ICT)进行,在该过程中,电子从主体共轭聚合物中被完全除去,并转移并保留在掺杂剂客体上。相反,对小共轭分子和低聚物进行化学掺杂通常会导致电荷转移络合物(CTC)的形成,这些电荷转移络合物具有明显的轨道重叠,并且在宿主和客体之间具有共享的电子密度。迄今为止,在掺杂的共轭聚合物中分数电荷转移的观察是相对罕见的,仅在可能难以实现的极端加工条件下发生,这是幸运的,因为四氯化碳的形成通常使每种掺杂剂产生较少的移动载流子。在这项工作中,我们使用P3HT和F 4的经典共轭聚合物/掺杂物对TCNQ演示了如何简单地在顺序处理中调整掺杂剂的浇铸溶剂如何从根本上改变这种经过充分研究的系统中掺杂的性质,从而实现可调谐CTC的生产。使用分别针对其对P3HT的低和高溶解度而选择的二氯甲烷和氯仿的溶剂共混物,我们表明聚合物掺杂CTC的相对量可以轻松控制在一个数量级上。增加掺杂剂溶剂混合物中的氯仿含量有利于生成四氯化碳,而增加二氯甲烷含量则导致采用更标准的ICT进行掺杂;这些结果使我们能够解释为什么CTC的形成在电荷转移盐中很常见,而在掺杂的共轭聚合物中却很少。我们还探讨了掺杂方法和P3HT薄膜结晶度在控制ICT和CTC形成的相对量中的作用。我们发现,使用蒸发掺杂和更高结晶度的材料会阻止CTC的形成,但是即使在最有利的情况下,具有高聚合物结晶度的蒸发掺杂,也总是会在一定程度上发生分数电荷转移。最后,我们证明了短暂的热退火可以将四氯化碳转化为整数电荷转移物质,这表明ICT是共轭聚合物中热力学上优选的掺杂机制,而分数电荷转移是动力学俘获的结果。有了这种了解,我们提供了一些指南,用于限制在顺序掺杂共轭聚合物过程中电荷转移络合物的出现,
更新日期:2021-04-13
中文翻译:

控制化学掺杂半导体聚合物中电荷转移络合物的形成
半导体聚合物的化学掺杂主要通过整数电荷转移(ICT)进行,在该过程中,电子从主体共轭聚合物中被完全除去,并转移并保留在掺杂剂客体上。相反,对小共轭分子和低聚物进行化学掺杂通常会导致电荷转移络合物(CTC)的形成,这些电荷转移络合物具有明显的轨道重叠,并且在宿主和客体之间具有共享的电子密度。迄今为止,在掺杂的共轭聚合物中分数电荷转移的观察是相对罕见的,仅在可能难以实现的极端加工条件下发生,这是幸运的,因为四氯化碳的形成通常使每种掺杂剂产生较少的移动载流子。在这项工作中,我们使用P3HT和F 4的经典共轭聚合物/掺杂物对TCNQ演示了如何简单地在顺序处理中调整掺杂剂的浇铸溶剂如何从根本上改变这种经过充分研究的系统中掺杂的性质,从而实现可调谐CTC的生产。使用分别针对其对P3HT的低和高溶解度而选择的二氯甲烷和氯仿的溶剂共混物,我们表明聚合物掺杂CTC的相对量可以轻松控制在一个数量级上。增加掺杂剂溶剂混合物中的氯仿含量有利于生成四氯化碳,而增加二氯甲烷含量则导致采用更标准的ICT进行掺杂;这些结果使我们能够解释为什么CTC的形成在电荷转移盐中很常见,而在掺杂的共轭聚合物中却很少。我们还探讨了掺杂方法和P3HT薄膜结晶度在控制ICT和CTC形成的相对量中的作用。我们发现,使用蒸发掺杂和更高结晶度的材料会阻止CTC的形成,但是即使在最有利的情况下,具有高聚合物结晶度的蒸发掺杂,也总是会在一定程度上发生分数电荷转移。最后,我们证明了短暂的热退火可以将四氯化碳转化为整数电荷转移物质,这表明ICT是共轭聚合物中热力学上优选的掺杂机制,而分数电荷转移是动力学俘获的结果。有了这种了解,我们提供了一些指南,用于限制在顺序掺杂共轭聚合物过程中电荷转移络合物的出现,

































 京公网安备 11010802027423号
京公网安备 11010802027423号