当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Three-Component Cascade Reaction of Benzaldehyde with Benzylamine and Hydroxylamine or Aniline: Synthesis of 1,2,4-Oxadiazoles and Quinazolines
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-03-05 , DOI: 10.1002/adsc.202001535 Chao Wang 1 , Xiyan Rui 1 , Dongjuan Si 1 , Rupeng Dai 1 , Yueyue Zhu 1 , Hongmei Wen 1 , Wei Li 1 , Jian Liu 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2021-03-05 , DOI: 10.1002/adsc.202001535 Chao Wang 1 , Xiyan Rui 1 , Dongjuan Si 1 , Rupeng Dai 1 , Yueyue Zhu 1 , Hongmei Wen 1 , Wei Li 1 , Jian Liu 1
Affiliation
|
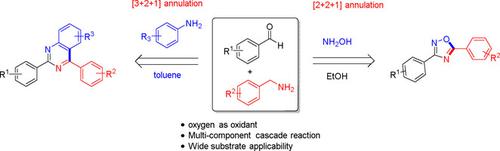
The analogous three-component synthesis strategy for substituted 1,2,4-oxadiazole and quinazoline derivatives from readily available benzaldehyde, benzylamine and hydroxylamine or aniline has been developed. Both the cascade reaction sequences involves nucleophilic addition of C−N bond, introduction a halogen donor, nucleophilic substitution and Cu(II)-catalyzed aerobic oxidation. This synthesis methodology demonstrated good yields, broad substrate scope and oxygen as a green oxidant. Thus, this synthesis protocol provides strategies for the construction of substituted 1,2,4-oxadiazole and quinazolines from readily and simple starting materials.
中文翻译:

铜催化苯甲醛与苄胺和羟胺或苯胺的三组分级联反应:合成 1,2,4-恶二唑和喹唑啉
已经开发了从容易获得的苯甲醛、苄胺和羟胺或苯胺中取代 1,2,4-恶二唑和喹唑啉衍生物的类似三组分合成策略。两个级联反应序列都涉及 CN 键的亲核加成、引入卤素供体、亲核取代和 Cu(II) 催化的有氧氧化。这种合成方法表现出良好的收率、广泛的底物范围和作为绿色氧化剂的氧气。因此,该合成方案为从容易和简单的起始材料构建取代的 1,2,4-恶二唑和喹唑啉提供了策略。
更新日期:2021-03-05
中文翻译:

铜催化苯甲醛与苄胺和羟胺或苯胺的三组分级联反应:合成 1,2,4-恶二唑和喹唑啉
已经开发了从容易获得的苯甲醛、苄胺和羟胺或苯胺中取代 1,2,4-恶二唑和喹唑啉衍生物的类似三组分合成策略。两个级联反应序列都涉及 CN 键的亲核加成、引入卤素供体、亲核取代和 Cu(II) 催化的有氧氧化。这种合成方法表现出良好的收率、广泛的底物范围和作为绿色氧化剂的氧气。因此,该合成方案为从容易和简单的起始材料构建取代的 1,2,4-恶二唑和喹唑啉提供了策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号