当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanochemical Redox: Calcination-free Synthesis of Ceria-hybrid Catalyst with Ultra-High Surface Area
ChemCatChem ( IF 3.8 ) Pub Date : 2021-03-04 , DOI: 10.1002/cctc.202100256 Siyang Nie 1 , Shize Yang 2 , Pengfei Zhang 3
ChemCatChem ( IF 3.8 ) Pub Date : 2021-03-04 , DOI: 10.1002/cctc.202100256 Siyang Nie 1 , Shize Yang 2 , Pengfei Zhang 3
Affiliation
|
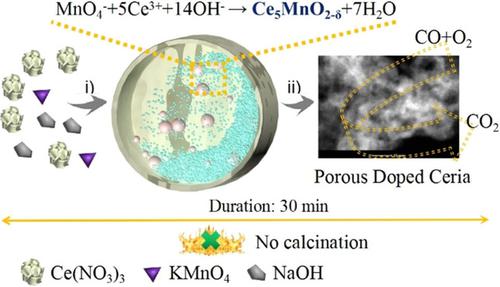
Transition metal-doped CeO2 (MCeOx) is of great importance in industrial catalysis. However, current synthetic methods often result in the separation of MOx phases, which significantly decreases their catalytic activity. Toward this end, the chemistry of mechanochemical redox was introduced to prepare Ce1-xMnxO2-δ catalyst. The redox behavior between MnO4− and Ce3+ could in situ produces atomically dispersed Ce1-xMnxO2-δ solid solution without calcination. Moreover, the mechanochemical synthesis endows Ce1-xMnxO2-δ a surface area (302 m2/g) that is much higher than the counterparts prepared by traditional methods, such as co-precipitation method (66 m2/g) CTAB-assisted precipitation method (122 m2/g) and sol-gel method (136 m2/g). Importantly, the well dispersed heteroatoms and high porosity afford doped ceria excellent activity and stability during CO oxidation. The reaction mechanism was finally explored by DFT calculation, which reveals that Mn and Cu dopants facilitate electron transfer and oxygen releasing.
中文翻译:

机械化学氧化还原:超煅烧二氧化铈杂化催化剂的无煅烧合成
过渡金属掺杂的CeO 2(MCeO x)在工业催化中非常重要。然而,当前的合成方法经常导致MO x相的分离,这大大降低了它们的催化活性。为此,引入了机械化学氧化还原化学以制备Ce 1-x Mn x O2 -δ催化剂。的MnO之间的氧化还原行为4 -和Ce 3+可以原位产生原子分散的Ce 1-x的Mn X ö 2-δ固体无需煅烧溶液。此外,机械化学合成赋予Ce 1-x Mnx O2 -δ的表面积(302 m 2 / g)比通过传统方法(如共沉淀法(66 m 2 / g)CTAB辅助沉淀法(122 m 2 / g)和溶胶-凝胶法(136 m 2 / g)。重要的是,良好分散的杂原子和高孔隙率使掺杂的二氧化铈在CO氧化过程中具有出色的活性和稳定性。最后通过DFT计算探索了反应机理,结果表明Mn和Cu掺杂剂促进了电子转移和氧释放。
更新日期:2021-03-04
中文翻译:

机械化学氧化还原:超煅烧二氧化铈杂化催化剂的无煅烧合成
过渡金属掺杂的CeO 2(MCeO x)在工业催化中非常重要。然而,当前的合成方法经常导致MO x相的分离,这大大降低了它们的催化活性。为此,引入了机械化学氧化还原化学以制备Ce 1-x Mn x O2 -δ催化剂。的MnO之间的氧化还原行为4 -和Ce 3+可以原位产生原子分散的Ce 1-x的Mn X ö 2-δ固体无需煅烧溶液。此外,机械化学合成赋予Ce 1-x Mnx O2 -δ的表面积(302 m 2 / g)比通过传统方法(如共沉淀法(66 m 2 / g)CTAB辅助沉淀法(122 m 2 / g)和溶胶-凝胶法(136 m 2 / g)。重要的是,良好分散的杂原子和高孔隙率使掺杂的二氧化铈在CO氧化过程中具有出色的活性和稳定性。最后通过DFT计算探索了反应机理,结果表明Mn和Cu掺杂剂促进了电子转移和氧释放。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号