当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Control of Mutagenic Impurities: Survey of Pharmaceutical Company Practices and a Proposed Framework for Industry Alignment
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-03-03 , DOI: 10.1021/acs.oprd.0c00517
Christopher J. Borths 1 , Mark D. Argentine 2 , John Donaubauer 3 , Eric L. Elliott 4 , Jared Evans 5 , Timothy Talbot Kramer 2 , Heewon Lee 6 , Rodney Parsons 7 , Jeffrey C. Roberts 2 , Gregory W. Sluggett 8 , Andrew Teasdale 9 , Michael Urquhart 10 , Ke Wang 8 , Ping Zhuang 11
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2021-03-03 , DOI: 10.1021/acs.oprd.0c00517
Christopher J. Borths 1 , Mark D. Argentine 2 , John Donaubauer 3 , Eric L. Elliott 4 , Jared Evans 5 , Timothy Talbot Kramer 2 , Heewon Lee 6 , Rodney Parsons 7 , Jeffrey C. Roberts 2 , Gregory W. Sluggett 8 , Andrew Teasdale 9 , Michael Urquhart 10 , Ke Wang 8 , Ping Zhuang 11
Affiliation
|
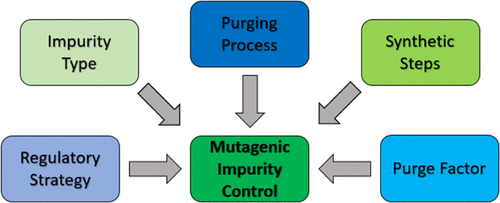
ICH M7 provides several risk-based control options to manage mutagenic and potentially mutagenic impurities (MI and PMIs) in the manufacture of pharmaceuticals. A Working Group in the International Consortium for Innovation and Quality in Pharmaceutical Development (IQ, www.iqconsortium.org) performed a survey of pharmaceutical manufacturers to gain insight into the use and regulatory acceptance of mutagenic impurity control strategies and control options across the industry. Information on the regulatory acceptance of ICH M7 control strategies was collected on late-stage clinical and commercial programs with regulatory feedback from the FDA, EMA, and PMDA. The data show a preference for ICH M7 Option 4 as it allows the utilization of process knowledge to reduce analytical testing without any compromise on patient safety. This approach appeared globally acceptable when appropriately applied. The survey data collected show that the ability of a manufacturing process to purge impurities is a strong indicator of purity control, and the data provide additional evidence that the purge factor calculation as proposed by Teasdale et al. produces a conservative prediction of the purging ability of a process. As such, the use of predicted purge factors and purge ratio assessments relative to the required process purge provides a sound framework for mutagenic impurity controls. Experimental purge data may be generated to support Option 4 strategies when predicted purge factors offer insufficient assurance for process control.
中文翻译:

诱变杂质的控制:制药公司实践调查和拟议的行业调整框架
ICH M7提供了几种基于风险的控制选项,以管理药品生产中的诱变和潜在诱变杂质(MI和PMI)。国际药品开发创新与质量联盟(IQ,www.iqconsortium.org)的一个工作组对药品制造商进行了调查,以了解整个行业中诱变杂质控制策略和控制选项的使用和监管接受情况。在后期临床和商业计划中收集了有关ICH M7控制策略的法规接受信息,并获得了FDA,EMA和PMDA的法规反馈。数据显示了对ICH M7 Option 4的偏爱,因为它允许利用过程知识来减少分析测试,而不会影响患者的安全。如果适当应用,此方法似乎在全球范围内都是可以接受的。收集的调查数据表明,制造过程中清除杂质的能力是纯度控制的有力指标,并且这些数据还提供了其他证据,证明Teasdale等人提出了清除因子的计算方法。保守地预测过程的清除能力。这样,相对于所需的工艺吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。收集的调查数据表明,制造过程中清除杂质的能力是纯度控制的有力指标,并且这些数据还提供了其他证据,证明Teasdale等人提出了清除因子的计算方法。保守地预测过程的清除能力。这样,相对于所需的工艺吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。收集的调查数据表明,制造过程中清除杂质的能力是纯度控制的有力指标,并且这些数据还提供了其他证据,证明Teasdale等人提出的清除因子的计算方法。保守地预测过程的清除能力。这样,相对于所需的工艺吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子无法为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。相对于所需的过程吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。相对于所需的过程吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。
更新日期:2021-04-16
中文翻译:

诱变杂质的控制:制药公司实践调查和拟议的行业调整框架
ICH M7提供了几种基于风险的控制选项,以管理药品生产中的诱变和潜在诱变杂质(MI和PMI)。国际药品开发创新与质量联盟(IQ,www.iqconsortium.org)的一个工作组对药品制造商进行了调查,以了解整个行业中诱变杂质控制策略和控制选项的使用和监管接受情况。在后期临床和商业计划中收集了有关ICH M7控制策略的法规接受信息,并获得了FDA,EMA和PMDA的法规反馈。数据显示了对ICH M7 Option 4的偏爱,因为它允许利用过程知识来减少分析测试,而不会影响患者的安全。如果适当应用,此方法似乎在全球范围内都是可以接受的。收集的调查数据表明,制造过程中清除杂质的能力是纯度控制的有力指标,并且这些数据还提供了其他证据,证明Teasdale等人提出了清除因子的计算方法。保守地预测过程的清除能力。这样,相对于所需的工艺吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。收集的调查数据表明,制造过程中清除杂质的能力是纯度控制的有力指标,并且这些数据还提供了其他证据,证明Teasdale等人提出了清除因子的计算方法。保守地预测过程的清除能力。这样,相对于所需的工艺吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。收集的调查数据表明,制造过程中清除杂质的能力是纯度控制的有力指标,并且这些数据还提供了其他证据,证明Teasdale等人提出的清除因子的计算方法。保守地预测过程的清除能力。这样,相对于所需的工艺吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子无法为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。相对于所需的过程吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。相对于所需的过程吹扫,使用预测的吹扫因子和吹扫比评估可为诱变杂质控制提供良好的框架。当预测的净化因子不能为过程控制提供足够的保证时,可能会生成实验净化数据以支持选项4的策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号