当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superalkali–Alkalide Interactions and Ion Pairing in Low-Polarity Solvents
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-03-04 , DOI: 10.1021/jacs.1c00115 René Riedel 1 , Andrew G Seel 2, 3 , Daniel Malko 1 , Daniel P Miller 4 , Brendan T Sperling 4 , Heungjae Choi 5 , Thomas F Headen 6 , Eva Zurek 7 , Adrian Porch 5 , Anthony Kucernak 1 , Nicholas C Pyper 8 , Peter P Edwards 3 , Anthony G M Barrett 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-03-04 , DOI: 10.1021/jacs.1c00115 René Riedel 1 , Andrew G Seel 2, 3 , Daniel Malko 1 , Daniel P Miller 4 , Brendan T Sperling 4 , Heungjae Choi 5 , Thomas F Headen 6 , Eva Zurek 7 , Adrian Porch 5 , Anthony Kucernak 1 , Nicholas C Pyper 8 , Peter P Edwards 3 , Anthony G M Barrett 1
Affiliation
|
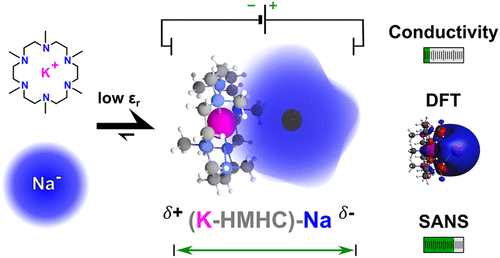
The nature of anionic alkali metals in solution is traditionally thought to be “gaslike” and unperturbed. In contrast to this noninteracting picture, we present experimental and computational data herein that support ion pairing in alkalide solutions. Concentration dependent ionic conductivity, dielectric spectroscopy, and neutron scattering results are consistent with the presence of superalkali–alkalide ion pairs in solution, whose stability and properties have been further investigated by DFT calculations. Our temperature dependent alkali metal NMR measurements reveal that the dynamics of the alkalide species is both reversible and thermally activated suggesting a complicated exchange process for the ion paired species. The results of this study go beyond a picture of alkalides being a “gaslike” anion in solution and highlight the significance of the interaction of the alkalide with its complex countercation (superalkali).
中文翻译:

低极性溶剂中的超级碱-碱化物相互作用和离子对
传统上认为溶液中阴离子碱金属的性质是“类气体”且不受干扰。与这种非相互作用的图片相反,我们在此提供了支持碱溶液中离子配对的实验和计算数据。浓度依赖性离子电导率、介电谱和中子散射结果与溶液中存在的超碱-碱离子对一致,其稳定性和性质已通过 DFT 计算进一步研究。我们的温度依赖性碱金属核磁共振测量表明,碱金属物质的动力学既是可逆的,又是热激活的,这表明离子配对物质的交换过程非常复杂。这项研究的结果超越了碱化物在溶液中作为“气体”阴离子的形象,并强调了碱化物与其复杂的抗衡阳离子(超碱)相互作用的重要性。
更新日期:2021-03-17
中文翻译:

低极性溶剂中的超级碱-碱化物相互作用和离子对
传统上认为溶液中阴离子碱金属的性质是“类气体”且不受干扰。与这种非相互作用的图片相反,我们在此提供了支持碱溶液中离子配对的实验和计算数据。浓度依赖性离子电导率、介电谱和中子散射结果与溶液中存在的超碱-碱离子对一致,其稳定性和性质已通过 DFT 计算进一步研究。我们的温度依赖性碱金属核磁共振测量表明,碱金属物质的动力学既是可逆的,又是热激活的,这表明离子配对物质的交换过程非常复杂。这项研究的结果超越了碱化物在溶液中作为“气体”阴离子的形象,并强调了碱化物与其复杂的抗衡阳离子(超碱)相互作用的重要性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号