当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Substrate-Specific Allosteric Effects on the Enhancement of CYP17A1 Lyase Efficiency by Cytochrome b5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-03-03 , DOI: 10.1021/jacs.1c00581 Yilin Liu 1 , Ilia G Denisov , Stephen G Sligar , James R Kincaid 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2021-03-03 , DOI: 10.1021/jacs.1c00581 Yilin Liu 1 , Ilia G Denisov , Stephen G Sligar , James R Kincaid 1
Affiliation
|
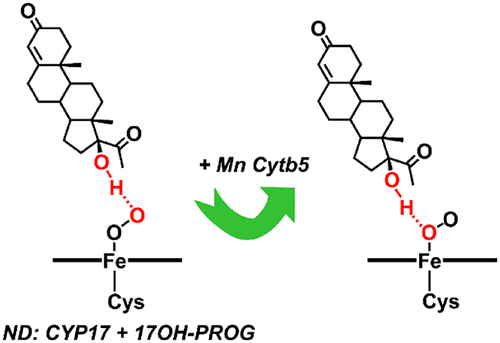
CYP17A1 is an essential human steroidogenic enzyme, which catalyzes two sequential reactions leading to the formation of androstenedione from progesterone and dehydroepiandrosterone from pregnenolone. The second reaction is the C17–C20 bond scission, which is strongly dependent on the presence of cytochrome b5 and displays a heretofore unexplained more pronounced acceleration when 17OH-progesteone (17OH-PROG) is a substrate. The origin of the stimulating effect of cytochrome b5 on C–C bond scission catalyzed by CYP17A1 is still debated as mostly due to either the acceleration of the electron transfer to the P450 oxy complex or allosteric effects of cytochrome b5 favoring active site conformations that promote lyase activity. Using resonance Raman spectroscopy, we compared the effect of Mn-substituted cytochrome b5 (Mn-Cytb5) on the oxy complex of CYP17A1 with both proteins co-incorporated in lipid nanodiscs. For CYP17A1 with 17OH-PROG, a characteristic shift of the Fe–O mode is observed in the presence of Mn-b5, indicating reorientation of a hydrogen bond between the 17OH group of the substrate from the terminal to the proximal oxygen atom of the Fe–O–O moiety, a configuration favorable for the lyase catalysis. For 17OH-pregnenolone, no such shift is observed, the favorable H-bonding orientation being present even without Mn-Cytb5. These new data provide a precise allosteric interpretation for the more pronounced acceleration seen for the 17OH-PROG substrate.
中文翻译:

细胞色素 b5 对 CYP17A1 裂解酶效率增强的底物特异性变构效应
CYP17A1 是一种人体必需的类固醇生成酶,它催化两个连续反应,导致孕酮生成雄烯二酮和孕烯醇酮生成脱氢表雄酮。第二个反应是 C17-C20 键断裂,它强烈依赖于细胞色素b 5的存在,当 17OH-孕酮 (17OH-PROG) 是底物时,它会表现出迄今为止无法解释的更明显的加速。细胞色素b 5对 CYP17A1 催化的 C-C 键断裂的刺激作用的起源仍然存在争议,主要是由于加速电子转移到 P450 氧复合物或细胞色素b 5的变构效应有利于促进裂解酶活性的活性位点构象。使用共振拉曼光谱,我们比较了 Mn 取代的细胞色素b 5 (Mn-Cyt b 5 ) 对 CYP17A1 的氧复合物的影响,这两种蛋白质共同掺入脂质纳米盘中。对于具有 17OH-PROG 的 CYP17A1,在 Mn- b 5存在下观察到 Fe-O 模式的特征转变,表明底物的 17OH 基团之间的氢键从末端到近端氧原子的重新取向Fe-O-O 部分,一种有利于裂解酶催化的构型。对于 17OH-孕烯醇酮,没有观察到这种转变,即使没有 Mn-Cyt b 5也存在有利的 H 键方向. 这些新数据为 17OH-PROG 底物更明显的加速提供了精确的变构解释。
更新日期:2021-03-17
中文翻译:

细胞色素 b5 对 CYP17A1 裂解酶效率增强的底物特异性变构效应
CYP17A1 是一种人体必需的类固醇生成酶,它催化两个连续反应,导致孕酮生成雄烯二酮和孕烯醇酮生成脱氢表雄酮。第二个反应是 C17-C20 键断裂,它强烈依赖于细胞色素b 5的存在,当 17OH-孕酮 (17OH-PROG) 是底物时,它会表现出迄今为止无法解释的更明显的加速。细胞色素b 5对 CYP17A1 催化的 C-C 键断裂的刺激作用的起源仍然存在争议,主要是由于加速电子转移到 P450 氧复合物或细胞色素b 5的变构效应有利于促进裂解酶活性的活性位点构象。使用共振拉曼光谱,我们比较了 Mn 取代的细胞色素b 5 (Mn-Cyt b 5 ) 对 CYP17A1 的氧复合物的影响,这两种蛋白质共同掺入脂质纳米盘中。对于具有 17OH-PROG 的 CYP17A1,在 Mn- b 5存在下观察到 Fe-O 模式的特征转变,表明底物的 17OH 基团之间的氢键从末端到近端氧原子的重新取向Fe-O-O 部分,一种有利于裂解酶催化的构型。对于 17OH-孕烯醇酮,没有观察到这种转变,即使没有 Mn-Cyt b 5也存在有利的 H 键方向. 这些新数据为 17OH-PROG 底物更明显的加速提供了精确的变构解释。































 京公网安备 11010802027423号
京公网安备 11010802027423号