当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Diastereotopic Group-Selective Intramolecular Aldol Reactions Initiated by Enantioselective Conjugate Silylation: Diastereodivergence Controlled by the Silicon Nucleophile
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-03-04 , DOI: 10.1021/acscatal.1c00436
Liangliang Zhang 1 , Martin Oestreich 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-03-04 , DOI: 10.1021/acscatal.1c00436
Liangliang Zhang 1 , Martin Oestreich 1
Affiliation
|
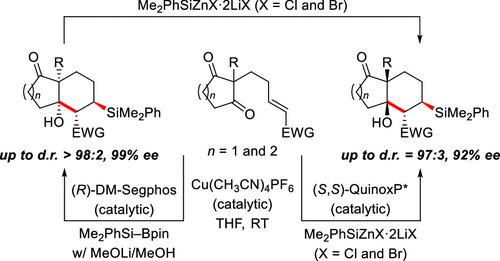
A reaction sequence consisting of enantioselective copper-catalyzed conjugate silylation and diastereotopic group-selective aldol cyclization is reported. The diastereoselectivity of the intramolecular aldol reaction depends on the silicon nucleophile used, either Me2PhSiZnX·2LiX (trans) or Me2PhSiBpin (cis). The more basic zinc reagent and as such the very basic reaction medium also enable thermodynamically driven cis-to-trans isomerization by a retro-aldol–aldol process. A broad range of electron-withdrawing groups including electron-deficient heterocycles is compatible with the different procedures developed.
中文翻译:

由对映选择性共轭硅烷化引发的非对映异构基团选择性分子内羟醛反应:由硅亲核试剂控制的非对映异构。
报道了由对映选择性铜催化的共轭甲硅烷基化和非对映异构基团选择性醛醇缩合环化反应组成的反应序列。分子内醛醇缩合反应的非对映选择性取决于所用的硅亲核试剂,Me 2 PhSiZnX·2LiX(反式)或Me 2 PhSiBpin(顺式)。碱性更强的锌试剂以及非常碱性的反应介质也可以通过逆醛醇-醛醇缩合工艺进行热力学驱动的顺式至反式异构化。广泛的吸电子基团,包括缺乏电子的杂环,与开发的不同方法兼容。
更新日期:2021-03-19
中文翻译:

由对映选择性共轭硅烷化引发的非对映异构基团选择性分子内羟醛反应:由硅亲核试剂控制的非对映异构。
报道了由对映选择性铜催化的共轭甲硅烷基化和非对映异构基团选择性醛醇缩合环化反应组成的反应序列。分子内醛醇缩合反应的非对映选择性取决于所用的硅亲核试剂,Me 2 PhSiZnX·2LiX(反式)或Me 2 PhSiBpin(顺式)。碱性更强的锌试剂以及非常碱性的反应介质也可以通过逆醛醇-醛醇缩合工艺进行热力学驱动的顺式至反式异构化。广泛的吸电子基团,包括缺乏电子的杂环,与开发的不同方法兼容。

































 京公网安备 11010802027423号
京公网安备 11010802027423号