当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Antitumor Application of Antiangiogenetic Gold Nanoclusters
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-03 , DOI: 10.1021/acsami.1c01164 Qirong Li 1, 2 , Ronghui Zhou 1 , Yue Sun 1 , Dexuan Xiao 1 , Mengting Liu 1 , Dan Zhao 1 , Shuanglin Peng 3 , Yu Chen 1, 2 , Yunfeng Lin 1, 4
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2021-03-03 , DOI: 10.1021/acsami.1c01164 Qirong Li 1, 2 , Ronghui Zhou 1 , Yue Sun 1 , Dexuan Xiao 1 , Mengting Liu 1 , Dan Zhao 1 , Shuanglin Peng 3 , Yu Chen 1, 2 , Yunfeng Lin 1, 4
Affiliation
|
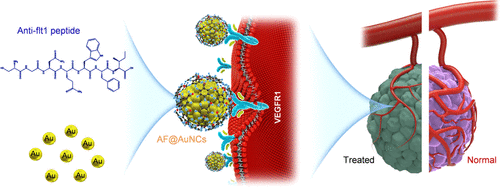
Conventional antiangiogenetic inhibitors suffered from poor delivery problems that result in unsatisfactory antitumor treatment efficacy. Although the liposomes or nanomaterial-based delivery systems can improve the therapeutic efficacy of antiangiogenic molecules, the assembly process is far too complex. Herein, a nanomaterial or a new nanodrug that could work without the help of a carrier and could be easily synthesized is needed. Au nanoclusters (AuNCs) are a kind of ideal nanostructures that could spontaneously enter into the cell and could be synthesized by a relatively easy one-pot method. Here, changing the traditional ligand glutathione (GSH) into an anti-Flt1 peptide (AF) has enriched the newly synthesized AF@AuNCs with targeted antiangiogenic properties. Based on the specific binding between AF and vascular endothelial growth factor receptor 1 (VEGFR1), the interaction between VEGFR1 and its ligands could be blocked. Furthermore, the expression of VEGFR2 could be downregulated. Compared with pure AF peptide- and GSH-participated AuNCs (GSH@AuNCs), AF@AuNCs were more effective in inhibiting both tube formation and migration of the endothelial cells in vitro. Furthermore, the in vivo chick embryo chorioallantoic membrane (CAM) experiment and antitumor experiment were conducted to further verify the enhanced antiangiogenesis and tumor inhibition effect of AF@AuNCs. Our findings provide promising evidence of a carrier-free nanodrug for tumors and other vascular hyperproliferative diseases.
中文翻译:

抗血管生成金纳米团簇的合成及抗肿瘤应用
传统的抗血管生成抑制剂存在递送问题,导致抗肿瘤治疗效果不理想。虽然基于脂质体或纳米材料的递送系统可以提高抗血管生成分子的治疗效果,但组装过程过于复杂。因此,需要一种无需载体即可发挥作用且易于合成的纳米材料或新型纳米药物。金纳米团簇(AuNCs)是一种理想的纳米结构,可以自发进入细胞,并且可以通过相对简单的一锅法合成。在这里,将传统的配体谷胱甘肽(GSH)改为抗Flt1肽(AF),丰富了新合成的具有靶向抗血管生成特性的AF@AuNC。基于AF与血管内皮生长因子受体1(VEGFR1)之间的特异性结合,可以阻断VEGFR1与其配体之间的相互作用。此外,VEGFR2的表达可能下调。与纯AF肽和GSH参与的AuNCs(GSH@AuNCs)相比,AF@AuNCs在体外更有效地抑制内皮细胞的管形成和迁移。此外,还进行了体内鸡胚绒毛尿囊膜(CAM)实验和抗肿瘤实验,进一步验证了AF@AuNCs增强的抗血管生成和肿瘤抑制作用。我们的研究结果为无载体纳米药物治疗肿瘤和其他血管过度增殖性疾病提供了有希望的证据。
更新日期:2021-03-17
中文翻译:

抗血管生成金纳米团簇的合成及抗肿瘤应用
传统的抗血管生成抑制剂存在递送问题,导致抗肿瘤治疗效果不理想。虽然基于脂质体或纳米材料的递送系统可以提高抗血管生成分子的治疗效果,但组装过程过于复杂。因此,需要一种无需载体即可发挥作用且易于合成的纳米材料或新型纳米药物。金纳米团簇(AuNCs)是一种理想的纳米结构,可以自发进入细胞,并且可以通过相对简单的一锅法合成。在这里,将传统的配体谷胱甘肽(GSH)改为抗Flt1肽(AF),丰富了新合成的具有靶向抗血管生成特性的AF@AuNC。基于AF与血管内皮生长因子受体1(VEGFR1)之间的特异性结合,可以阻断VEGFR1与其配体之间的相互作用。此外,VEGFR2的表达可能下调。与纯AF肽和GSH参与的AuNCs(GSH@AuNCs)相比,AF@AuNCs在体外更有效地抑制内皮细胞的管形成和迁移。此外,还进行了体内鸡胚绒毛尿囊膜(CAM)实验和抗肿瘤实验,进一步验证了AF@AuNCs增强的抗血管生成和肿瘤抑制作用。我们的研究结果为无载体纳米药物治疗肿瘤和其他血管过度增殖性疾病提供了有希望的证据。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号