当前位置:
X-MOL 学术
›
FEBS Open Bio
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The pyruvate:ferredoxin oxidoreductase of the thermophilic acetogen, Thermoanaerobacter kivui
FEBS Open Bio ( IF 2.8 ) Pub Date : 2021-03-04 , DOI: 10.1002/2211-5463.13136 Alexander Katsyv 1 , Marie Charlotte Schoelmerich 1 , Mirko Basen 1 , Volker Müller 1
FEBS Open Bio ( IF 2.8 ) Pub Date : 2021-03-04 , DOI: 10.1002/2211-5463.13136 Alexander Katsyv 1 , Marie Charlotte Schoelmerich 1 , Mirko Basen 1 , Volker Müller 1
Affiliation
|
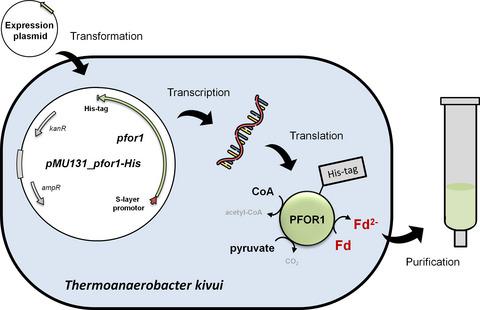
Pyruvate:ferredoxin oxidoreductase (PFOR) is a key enzyme in bacterial anaerobic metabolism. Since a low‐potential ferredoxin (Fd2−) is used as electron carrier, PFOR allows for hydrogen evolution during heterotrophic growth as well as pyruvate synthesis during lithoautotrophic growth. The thermophilic acetogenic model bacterium Thermoanaerobacter kivui can use both modes of lifestyle, but the nature of the PFOR in this organism was previously unestablished. Here, we have isolated PFOR to apparent homogeneity from cells grown on glucose. Peptide mass fingerprinting revealed that it is encoded by pfor1. PFOR uses pyruvate as an electron donor and methylene blue (1.8 U·mg−1) and ferredoxin (Fd; 27.2 U·mg−1) as electron acceptors, and the reaction is dependent on thiamine pyrophosphate, pyruvate, coenzyme A, and Fd. The pH and temperature optima were 7.5 and 66 °C, respectively. We detected 13.6 mol of iron·mol of protein−1, consistent with the presence of three predicted [4Fe–4S] clusters. The ability to provide reduced Fd makes PFOR an interesting auxiliary enzyme for enzyme assays. To simplify and speed up the purification procedure, we established a protocol for homologous protein production in T. kivui. Therefore, pfor1 was cloned and expressed in T. kivui and the encoded protein containing a genetically engineered His‐tag was purified in only two steps to apparent homogeneity. The homologously produced PFOR1 had the same properties as the enzyme from T. kivui. The enzyme can be used as auxiliary enzyme in enzymatic assays that require reduced Fd as electron donor, such as electron‐bifurcating enzymes, to keep a constant level of reduced Fd.
中文翻译:

丙酮酸:嗜热产乙酸菌的铁氧还蛋白氧化还原酶,Thermoanaerobacter kivui
丙酮酸:铁氧还蛋白氧化还原酶(PFOR)是细菌厌氧代谢的关键酶。由于使用低电位铁氧还蛋白 (Fd 2- ) 作为电子载体,PFOR 允许在异养生长期间析氢以及在岩石自养生长期间合成丙酮酸。嗜热产乙酸模型细菌Thermoanaerobacter kivui可以使用这两种生活方式,但以前尚未确定该生物体中 PFOR 的性质。在这里,我们已经将 PFOR 从在葡萄糖上生长的细胞中分离到明显的同质性。肽质量指纹显示它是由pfor1编码的。PFOR 使用丙酮酸作为电子供体和亚甲蓝 (1.8 U·mg -1 ) 和铁氧还蛋白 (Fd; 27.2 U·mg -1) 作为电子受体,反应依赖于焦磷酸硫胺素、丙酮酸、辅酶 A 和 Fd。最佳 pH 值和温度分别为 7.5 和 66 °C。我们检测到 13.6 mol 铁·mol 蛋白质-1,与三个预测的 [4Fe-4S] 簇的存在一致。提供减少 Fd 的能力使 PFOR 成为一种有趣的酶测定辅助酶。为了简化和加快纯化过程,我们建立了在T. kivui 中生产同源蛋白质的协议。因此,pfor1被克隆并在T. kivui 中表达并且编码的含有基因工程 His 标签的蛋白质仅通过两步纯化,达到明显的同质性。同源产生的 PFOR1 与来自T. kivui的酶具有相同的特性。该酶可在需要还原 Fd 作为电子供体的酶促测定中用作辅助酶,例如电子分叉酶,以保持还原 Fd 的恒定水平。
更新日期:2021-05-03
中文翻译:

丙酮酸:嗜热产乙酸菌的铁氧还蛋白氧化还原酶,Thermoanaerobacter kivui
丙酮酸:铁氧还蛋白氧化还原酶(PFOR)是细菌厌氧代谢的关键酶。由于使用低电位铁氧还蛋白 (Fd 2- ) 作为电子载体,PFOR 允许在异养生长期间析氢以及在岩石自养生长期间合成丙酮酸。嗜热产乙酸模型细菌Thermoanaerobacter kivui可以使用这两种生活方式,但以前尚未确定该生物体中 PFOR 的性质。在这里,我们已经将 PFOR 从在葡萄糖上生长的细胞中分离到明显的同质性。肽质量指纹显示它是由pfor1编码的。PFOR 使用丙酮酸作为电子供体和亚甲蓝 (1.8 U·mg -1 ) 和铁氧还蛋白 (Fd; 27.2 U·mg -1) 作为电子受体,反应依赖于焦磷酸硫胺素、丙酮酸、辅酶 A 和 Fd。最佳 pH 值和温度分别为 7.5 和 66 °C。我们检测到 13.6 mol 铁·mol 蛋白质-1,与三个预测的 [4Fe-4S] 簇的存在一致。提供减少 Fd 的能力使 PFOR 成为一种有趣的酶测定辅助酶。为了简化和加快纯化过程,我们建立了在T. kivui 中生产同源蛋白质的协议。因此,pfor1被克隆并在T. kivui 中表达并且编码的含有基因工程 His 标签的蛋白质仅通过两步纯化,达到明显的同质性。同源产生的 PFOR1 与来自T. kivui的酶具有相同的特性。该酶可在需要还原 Fd 作为电子供体的酶促测定中用作辅助酶,例如电子分叉酶,以保持还原 Fd 的恒定水平。































 京公网安备 11010802027423号
京公网安备 11010802027423号