当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Hydride Transfer from Catalytic Metal-Free Hydride Donors to CO2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-03-02 , DOI: 10.1021/acs.jpclett.0c03662
Ravindra B. Weerasooriya 1, 2 , Jonathan L. Gesiorski 1, 2 , Abdulaziz Alherz 3 , Stefan Ilic 4 , George N. Hargenrader 1, 2 , Charles B. Musgrave 3, 5 , Ksenija D. Glusac 1, 2
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2021-03-02 , DOI: 10.1021/acs.jpclett.0c03662
Ravindra B. Weerasooriya 1, 2 , Jonathan L. Gesiorski 1, 2 , Abdulaziz Alherz 3 , Stefan Ilic 4 , George N. Hargenrader 1, 2 , Charles B. Musgrave 3, 5 , Ksenija D. Glusac 1, 2
Affiliation
|
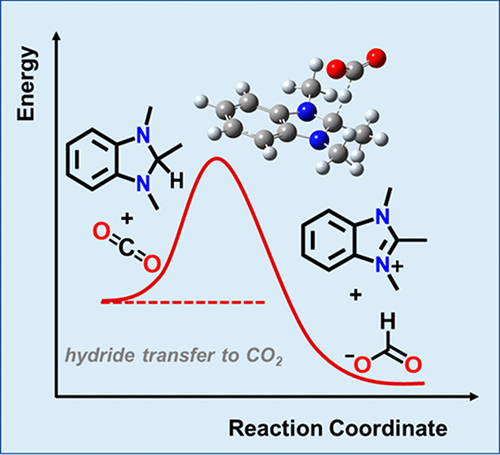
Selective reduction of CO2 to formate represents an ongoing challenge in photoelectrocatalysis. To provide mechanistic insights, we investigate the kinetics of hydride transfer (HT) from a series of metal-free hydride donors to CO2. The observed dependence of experimental and calculated HT barriers on the thermodynamic driving force was modeled by using the Marcus hydride transfer formalism to obtain the insights into the effect of reorganization energies on the reaction kinetics. Our results indicate that even if the most ideal hydride donor were discovered, the HT to CO2 would exhibit sluggish kinetics (<100 turnovers per second at −0.1 eV driving force), indicating that the conventional HT may not be an appropriate mechanism for solar conversion of CO2 to formate. We propose that the conventional HT mechanism should not be considered for CO2 reduction catalysis and argue that the orthogonal HT mechanism, previously proposed to address thermodynamic limitations of this reaction, may also lead to lower kinetic barriers for CO2 reduction to formate.
中文翻译:

氢化物从无催化金属氢化物供体向CO 2转移的动力学
将CO 2选择性还原成甲酸酯代表了光电催化中的一项持续挑战。为了提供机械方面的见解,我们研究了一系列从无金属氢化物供体到CO 2的氢化物转移(HT)的动力学。使用Marcus氢化物转移形式主义对实验和计算得出的HT壁垒对热力学驱动力的依赖关系进行建模,以了解重组能量对反应动力学的影响。我们的结果表明,即使发现了最理想的氢化物供体,HT转化为CO 2也会表现出缓慢的动力学(在-0.1 eV驱动力下,<100周转/秒),这表明常规HT可能不是太阳能的合适机理。一氧化碳转化率2进行格式化。我们提出不应该考虑将传统的HT机理用于CO 2还原催化,并提出先前提出的用于解决该反应热力学局限性的正交HT机理也可能导致降低CO 2还原成甲酸酯的动力学障碍。
更新日期:2021-03-11
中文翻译:

氢化物从无催化金属氢化物供体向CO 2转移的动力学
将CO 2选择性还原成甲酸酯代表了光电催化中的一项持续挑战。为了提供机械方面的见解,我们研究了一系列从无金属氢化物供体到CO 2的氢化物转移(HT)的动力学。使用Marcus氢化物转移形式主义对实验和计算得出的HT壁垒对热力学驱动力的依赖关系进行建模,以了解重组能量对反应动力学的影响。我们的结果表明,即使发现了最理想的氢化物供体,HT转化为CO 2也会表现出缓慢的动力学(在-0.1 eV驱动力下,<100周转/秒),这表明常规HT可能不是太阳能的合适机理。一氧化碳转化率2进行格式化。我们提出不应该考虑将传统的HT机理用于CO 2还原催化,并提出先前提出的用于解决该反应热力学局限性的正交HT机理也可能导致降低CO 2还原成甲酸酯的动力学障碍。

































 京公网安备 11010802027423号
京公网安备 11010802027423号