当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regioselective Radical Amino-Functionalizations of Allyl Alcohols via Dual Catalytic Cross-Coupling
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-03-03 , DOI: 10.1021/acscatal.1c00404 Zuxiao Zhang 1 , Duong T Ngo 1 , David A Nagib 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2021-03-03 , DOI: 10.1021/acscatal.1c00404 Zuxiao Zhang 1 , Duong T Ngo 1 , David A Nagib 1
Affiliation
|
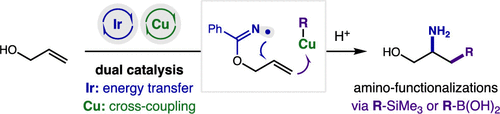
The regioselective amination and cross-coupling of a range of nucleophiles with allyl alcohols has been enabled by a dual catalytic strategy. This approach entails the combined action of an Ir photocatalyst that enables mild access to N-radicals via an energy transfer mechanism, as well as a Cu complex that intercepts the ensuing alkyl radical upon cyclization. Merger of this Cu-catalyzed cross-coupling enables a broad range of nucleophiles (e.g., CN, SCN, N3, vinyl, allyl) to engage in radical amino-functionalizations of olefins. Notably, stereo, regio, and kinetic probes provide insights into the nature of this Cu-based radical interception.
中文翻译:

通过双催化交叉偶联对烯丙醇进行区域选择性自由基氨基官能化
通过双重催化策略实现了一系列亲核试剂与烯丙醇的区域选择性胺化和交叉偶联。这种方法需要 Ir 光催化剂的联合作用,通过能量转移机制可以温和地接近 N-自由基,以及在环化时拦截随后的烷基自由基的 Cu 配合物。这种Cu 催化的交叉偶联的合并使广泛的亲核试剂(例如CN、SCN、N 3、乙烯基、烯丙基)能够参与烯烃的自由基氨基官能化。值得注意的是,立体、区域和动力学探针提供了对这种基于 Cu 的自由基拦截性质的见解。
更新日期:2021-03-19
中文翻译:

通过双催化交叉偶联对烯丙醇进行区域选择性自由基氨基官能化
通过双重催化策略实现了一系列亲核试剂与烯丙醇的区域选择性胺化和交叉偶联。这种方法需要 Ir 光催化剂的联合作用,通过能量转移机制可以温和地接近 N-自由基,以及在环化时拦截随后的烷基自由基的 Cu 配合物。这种Cu 催化的交叉偶联的合并使广泛的亲核试剂(例如CN、SCN、N 3、乙烯基、烯丙基)能够参与烯烃的自由基氨基官能化。值得注意的是,立体、区域和动力学探针提供了对这种基于 Cu 的自由基拦截性质的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号